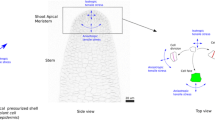
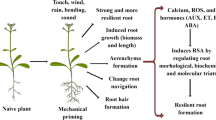
Abstract
Mechanical stimuli, such as touch, bending, gravity, and wounding, influence plant growth and development through the activation of intracellular signaling pathways and gene expression. Therefore, mechanosensing and mechanotransduction are of vital importance and have been attracting the attention of many plant scientists for nearly 150 years. Based on recent molecular and cellular approaches, candidates for mechanosensors have been discovered. These include mechanosensitive (MS) channels, such as MscS-like (MSL) proteins, mid1-complementing activities (MCAs), and reduced hyperosmolality-induced [Ca2+]i increase 1 (OSCA1), which generate intracellular ionic signals and receptor-like kinases that trigger the activation of regulatory proteins or enzymes, including Ca2+-binding proteins, protein kinases, protein phosphatases, and transcription factors. Other possible groups of mechanosensors are intracellular filamentous structures in the cytoskeleton, such as microtubules and actin filaments, which may directly act as sensors for the deformation of intracellular structures. In this chapter, we discuss the mechanisms by which plants sense and respond to mechanical stimuli by focusing on mechanosensors along with their downstream signaling molecules, such as auxin and reactive oxygen species (ROS).
M. Toyota and T. Furuichi contributed equally to this chapter.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- CBLs:
-
Calcineurin B-like proteins
- CICR:
-
Ca2+-induced Ca2+ release
- CIPKs:
-
CBL-interacting protein kinases
- FtsZ:
-
Filamentous temperature-sensitive Z
- GLR:
-
Glutamate receptor-like channels
- InsP3:
-
Inositol 1,4,5-trisphosphate
- MCA1:
-
mid1-complementing activity 1
- MCAs:
-
mid1-complementing activities
- MS:
-
Mechanosensitive
- MscL:
-
Mechanosensitive channel of large conductance
- MscS:
-
Mechanosensitive channel of small conductance
- MSL:
-
MscS-like
- OSCA1:
-
Reduced hyperosmolality-induced [Ca2+]i increase 1
- RAL:
-
Rapid alkalinization factor
- ROS:
-
Reactive oxygen species
- SA:
-
Stretch-activated
- TM:
-
Transmembrane
- TPC1:
-
Two-pore channel 1
References
Allen GJ, Chu SP, Harrington CL, Schumacher K, Hoffmann T, Tang YY et al (2001) A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411:1053–1057
Anishkin A, Loukin SH, Teng J, Kung C (2014) Feeling the hidden mechanical forces in lipid bilayer is an original sense. Proc Natl Acad Sci USA 111:7898–7905
Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J (2000) Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 406:512–515
Asai N, Nishioka T, Takabayashi J, Furuichi T (2009) Plant volatiles regulate the activities of Ca2+-permeable channels and promote cytoplasmic calcium transients in Arabidopsis leaf cells. Plant Signal Behav 4:294–300
Baskin TI (2001) On the alignment of cellulose microfibrils by cortical microtubules: a review and a model. Protoplasma 215:150–171
Baskin TI (2005) Anisotropic expansion of the plant cell wall. Annu Rev Cell Dev Biol 21:203–222
Baskin TI, Wilson JE, Cork A, Williamson RE (1994) Morphology and microtubule organization in Arabidopsis roots exposed to oryzalin or taxol. Plant Cell Physiol 35:935–942
Berridge MJ (1997) The AM and FM of calcium signalling. Nature 386:759–760
Blancaflor EB, Hasenstein KH (1993) Organization of cortical microtubules in graviresponding maize roots. Planta 191:231–237
Booth IR, Blount P (2012) The MscS and MscL families of mechanosensitive channels act as microbial emergency release valves. J Bacteriol 194:4802–4809
Bowman CL, Gottlieb PA, Suchyna TM, Murphy YK, Sachs F (2007) Mechanosensitive ion channels and the peptide inhibitor GsMTx-4: history, properties, mechanisms and pharmacology. Toxicon 49:249–270
Braam J, Davis RW (1990) Rain-, wind-, and touch-induced expression of calmodulin and calmodulin-related genes in Arabidopsis. Cell 60:357–364
Brohawn SG (2015) How ion channels sense mechanical force: insights from mechanosensitive K2P channels TRAAK, TREK1, and TREK2. Ann NY Acad Sci 1352:20–32
Burian A, Ludynia M, Uyttewaal M, Traas J, Boudaoud A, Hamant O et al (2013) A correlative microscopy approach relates microtubule behaviour, local organ geometry, and cell growth at the Arabidopsis shoot apical meristem. J Exp Bot 64:5753–5767
Carpita NC, Gibeaut DM (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the walls during growth. Plant J 3:1–30
Chen Y, Simasko SM, Niggel J, Sigurdson WJ, Sachs F (1996) Ca2+ uptake in GH3 cells during hypotonic swelling: the sensory role of stretch-activated ion channels. Am J Physiol 270:C1790–C1798
Ciesielski T (1871) In Abwärtskrümmung der Wurzel. Inaug Dissert, Breslau
Cosgrove DJ, Hedrich R (1991) Stretch-activated chloride, potassium, and calcium channels coexisting in plasma membranes of guard cells of Vicia faba L. Planta 186:143–153
Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ et al (2010) Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330:55–60
Darwin C (1880) The power of movement in plants. John Murray, London
Darwin C (1888) The movements and habits of climbing plants. John Murray, London
Denness L, McKenna JF, Segonzac C, Wormit A, Madhou P, Bennett M et al (2011) Cell wall damage-induced lignin biosynthesis is regulated by a reactive oxygen species- and jasmonic acid-dependent process in Arabidopsis. Plant Physiol 156:1364–1374
Ding JP, Pickard BG (1993) Mechanosensory calcium-selective cation channels in epidermal cells. Plant J 3:83–110
Dutta R, Robinson KR (2004) Identification and characterization of stretch-activated ion channels in pollen protoplasts. Plant Physiol 135:1398–1406
Evans MJ, Choi WG, Gilroy S, Morris RJ (2016) A ROS-assisted calcium wave dependent on the AtRBOHD NADPH oxidase and TPC1 cation channel propagates the systemic response to salt stress. Plant Physiol 171:1771–1784
Falke LC, Edwards KL, Pickard BG, Misler S (1988) A stretch-activated anion channel in tobacco protoplasts. FEBS Lett 237:141–144
Farmer EE, Gasperini D, Acosta IF (2014) The squeeze cell hypothesis for the activation of jasmonate synthesis in response to wounding. New Phytol 204:282–288
Fischer K, Schopfer P (1997) Interaction of auxin, light, and mechanical stress in orienting microtubules in relation to tropic curvature in the epidermis of maize coleoptiles. Protoplasma 196:108–116
Furuichi T, Lida H, Sokabe M, Tatsumi H (2012) Expression of arabidopsis MCA1 enhanced mechanosensitive channel activity in the Xenopus laevis oocyte plasma membrane. Plant Signal Behav 7:1022–1026
Ge J, Li W, Zhao Q, Li N, Chen M, Zhi P et al (2015) Architecture of the mammalian mechanosensitive Piezo1 channel. Nature 527:64–69
Gilroy S, Suzuki N, Miller G, Choi WG, Toyota M, Devireddy AR et al (2014) A tidal wave of signals: calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci 19:623–630
Groover A (2016) Gravitropisms and reaction woods of forest trees—evolution, functions and mechanisms. New Phytol 211:790–802
Guo Y, Halfter U, Ishitani M, Zhu JK (2001) Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell 13:1383–1400
Gutierrez R, Lindeboom JJ, Paredez AR, Emons AM, Ehrhardt DW (2009) Arabidopsis cortical microtubules position cellulose synthase delivery to the plasma membrane and interact with cellulose synthase trafficking compartments. Nat Cell Biol 11:797–806
Hamant O, Heisler MG, Jonsson H, Krupinski P, Uyttewaal M, Bokov P et al (2008) Developmental patterning by mechanical signals in Arabidopsis. Science 322:1650–1655
Hamant O, Moulia B (2016) How do plants read their own shapes? New Phytol 212:333–337
Hamilton ES, Jensen GS, Maksaev G, Katims A, Sherp AM, Haswell ES (2015) Mechanosensitive channel MSL8 regulates osmotic forces during pollen hydration and germination. Science 350:438–441
Haruta M, Sabat G, Stecker K, Minkoff BB, Sussman MR (2014) A peptide hormone and its receptor protein kinase regulate plant cell expansion. Science 343:408–411
Haswell ES, Meyerowitz EM (2006) MscS-like proteins control plastid size and shape in Arabidopsis thaliana. Curr Biol 16:1–11
Haswell ES, Peyronnet R, Barbier-Brygoo H, Meyerowitz EM, Frachisse JM (2008) Two MscS homologs provide mechanosensitive channel activities in the Arabidopsis root. Curr Biol 18:730–734
Hayakawa K, Tatsumi H, Sokabe M (2008) Actin stress fibers transmit and focus force to activate mechanosensitive channels. J Cell Sci 121:496–503
Himmelspach R, Wymer CL, Lloyd CW, Nick P (1999) Gravity-induced reorientation of cortical microtubules observed in vivo. Plant J 18:449–453
Hoson T, Soga K (2003) New aspects of gravity responses in plant cells. Int Rev Cytol 229:209–244
Hou G, Kramer VL, Wang YS, Chen R, Perbal G, Gilroy S et al (2004) The promotion of gravitropism in Arabidopsis roots upon actin disruption is coupled with the extended alkalinization of the columella cytoplasm and a persistent lateral auxin gradient. Plant J 39:113–125
Hou G, Mohamalawari DR, Blancaflor EB (2003) Enhanced gravitropism of roots with a disrupted cap actin cytoskeleton. Plant Physiol 131:1360–1373
Iida H (2014) Mugifumi, a beneficial farm work of adding mechanical stress by treading to wheat and barley seedlings. Front Plant Sci 5:453
Ikushima T, Shimmen T (2005) Mechano-sensitive orientation of cortical microtubules during gravitropism in azuki bean epicotyls. J Plant Res 118:19–26
Jeandroz S, Lamotte O, Astier J, Rasul S, Trapet P, Besson-Bard A et al (2013) There’s more to the picture than meets the eye: nitric oxide cross talk with Ca2+ signaling. Plant Physiol 163:459–470
Joo JH, Bae YS, Lee JS (2001) Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol 126:1055–1060
Kamano S, Kume S, Iida K, Lei KJ, Nakano M, Nakayama Y et al (2015) Transmembrane topologies of Ca2+-permeable mechanosensitive channels MCA1 and MCA2 in Arabidopsis thaliana. J Biol Chem 290:30901–30909
Kiep V, Vadassery J, Lattke J, Maass JP, Boland W, Peiter E et al (2015) Systemic cytosolic Ca2+ elevation is activated upon wounding and herbivory in Arabidopsis. New Phytol 207:996–1004
Kim BG, Waadt R, Cheong YH, Pandey GK, Dominguez-Solis JR, Schultke S et al (2007) The calcium sensor CBL10 mediates salt tolerance by regulating ion homeostasis in Arabidopsis. Plant J 52:473–484
Kloda A, Martinac B (2002) Common evolutionary origins of mechanosensitive ion channels in Archaea, Bacteria and cell-walled Eukarya. Archaea 1:35–44
Knight MR, Campbell AK, Smith SM, Trewavas AJ (1991) Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352:524–526
Knight MR, Smith SM, Trewavas AJ (1992) Wind-induced plant motion immediately increases cytosolic calcium. Proc Natl Acad Sci USA 89:4967–4971
Krieg M, Dunn AR, Goodman MB (2015) Mechanical systems biology of C. elegans touch sensation. BioEssays 37:335–344
Kurusu T, Kuchitsu K, Nakano M, Nakayama Y, Iida H (2013) Plant mechanosensing and Ca2+ transport. Trends Plant Sci 18:227–233
Kurusu T, Iida H, Kuchitsu K (2012a) Roles of a putative mechanosensitive plasma membrane Ca2+-permeable channel OsMCA1 in generation of reactive oxygen species and hypo-osmotic signaling in rice. Plant Signal Behav 7:796–798
Kurusu T, Nishikawa D, Yamazaki Y, Gotoh M, Nakano M, Hamada H et al (2012b) Plasma membrane protein OsMCA1 is involved in regulation of hypo-osmotic shock-induced Ca2+ influx and modulates generation of reactive oxygen species in cultured rice cells. BMC Plant Biol 12:11
Kurusu T, Yamanaka T, Nakano M, Takiguchi A, Ogasawara Y, Hayashi T et al (2012c) Involvement of the putative Ca2+-permeable mechanosensitive channels, NtMCA1 and NtMCA2, in Ca2+ uptake, Ca2+-dependent cell proliferation and mechanical stress-induced gene expression in tobacco (Nicotiana tabacum) BY-2 cells. J Plant Res 125:555–568
Laluk K, Prasad KV, Savchenko T, Celesnik H, Dehesh K, Levy M et al (2012) The calmodulin-binding transcription factor SIGNAL RESPONSIVE1 is a novel regulator of glucosinolate metabolism and herbivory tolerance in Arabidopsis. Plant Cell Physiol 53:2008–2015
Lee CP, Maksaev G, Jensen GS, Murcha MW, Wilson ME, Fricker M et al (2016) MSL1 is a mechanosensitive ion channel that dissipates mitochondrial membrane potential and maintains redox homeostasis in mitochondria during abiotic stress. Plant J 88:809–825
Levina N, Totemeyer S, Stokes NR, Louis P, Jones MA, Booth IR (1999) Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J 18:1730–1737
Li Y, Yuan F, Wen Z, Li Y, Wang F, Zhu T et al (2015) Genome-wide survey and expression analysis of the OSCA gene family in rice. BMC Plant Biol 15:261
Liu Z, Cheng Q, Sun Y, Dai H, Song G, Guo Z et al (2015) A SNP in OsMCA1 responding for a plant architecture defect by deactivation of bioactive GA in rice. Plant Mol Biol 87:17–30
Maksaev G, Haswell ES (2012) MscS-Like10 is a stretch-activated ion channel from Arabidopsis thaliana with a preference for anions. Proc Natl Acad Sci USA 109:19015–19020
Maple J, Chua NH, Moller SG (2002) The topological specificity factor AtMinE1 is essential for correct plastid division site placement in Arabidopsis. Plant J 31:269–277
Messerli MA, Danuser G, Robinson KR (1999) Pulsatile influxes of H+, K+ and Ca2+ lag growth pulses of Lilium longiflorum pollen tubes. J Cell Sci 112:1497–1509
Monshausen GB, Bibikova TN, Weisenseel MH, Gilroy S (2009) Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabidopsis roots. Plant Cell 21:2341–2356
Moulia B, Coutand C, Julien JL (2015) Mechanosensitive control of plant growth: bearing the load, sensing, transducing, and responding. Front Plant Sci 6:52
Mousavi SA, Chauvin A, Pascaud F, Kellenberger S, Farmer EE (2013) GLUTAMATE RECEPTOR-LIKE genes mediate leaf-to-leaf wound signalling. Nature 500:422–426
Nakagawa Y, Katagiri T, Shinozaki K, Qi Z, Tatsumi H, Furuichi T et al (2007) Arabidopsis plasma membrane protein crucial for Ca2+ influx and touch sensing in roots. Proc Natl Acad Sci USA 104:3639–3644
Nakamura M, Toyota M, Tasaka M, Morita MT (2011) An Arabidopsis E3 Ligase, SHOOT GRAVITROPISM9, Modulates the Interaction between Statoliths and F-Actin in Gravity Sensing. Plant Cell 23:1830–1848
Nakano M, Iida K, Nyunoya H, Iida H (2011) Determination of structural regions important for Ca(2+) uptake activity in Arabidopsis MCA1 and MCA2 expressed in yeast. Plant Cell Physiol 52:1915–1930
Nakayama Y, Fujiu K, Sokabe M, Yoshimura K (2007) Molecular and electrophysiological characterization of a mechanosensitive channel expressed in the chloroplasts of Chlamydomonas. Proc Natl Acad Sci USA 104:5883–5888
Nick P, Bergfeld R, Schafer E, Schopfer P (1990) Unilateral reorientation of microtubules at the outer epidermal wall during photo- and gravitropic curvature of maize coleoptiles and sunflower hypocotyls. Planta 181:162–168
Palmieri M, Kiss JZ (2005) Disruption of the F-actin cytoskeleton limits statolith movement in Arabidopsis hypocotyls. J Exp Bot 56:2539–2550
Paredez AR, Somerville CR, Ehrhardt DW (2006) Visualization of cellulose synthase demonstrates functional association with microtubules. Science 312:1491–1495
Perera IY, Hung CY, Brady S, Muday GK, Boss WF (2006) A universal role for inositol 1,4,5-trisphosphate-mediated signaling in plant gravitropism. Plant Physiol 140:746–760
Plieth C, Trewavas AJ (2002) Reorientation of seedlings in the earth’s gravitational field induces cytosolic calcium transients. Plant Physiol 129:786–796
Qiu QS, Guo Y, Dietrich MA, Schumaker KS, Zhu JK (2002) Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc Natl Acad Sci USA 99:8436–8441
Rigo G, Ayaydin F, Tietz O, Zsigmond L, Kovacs H, Pay A et al (2013) Inactivation of plasma membrane-localized CDPK-RELATED KINASE5 decelerates PIN2 exocytosis and root gravitropic response in Arabidopsis. Plant Cell 25:1592–1608
Robinson S, Burian A, Couturier E, Landrein B, Louveaux M, Neumann ED et al (2013) Mechanical control of morphogenesis at the shoot apex. J Exp Bot 64:4729–4744
Rosa M, Abraham-Juarez MJ, Lewis MW, Fonseca JP, Tian W, Ramirez V et al (2017) The Maize MID-COMPLEMENTING ACTIVITY homolog CELL NUMBER REGULATOR13/NARROW ODD DWARF coordinates organ growth and tissue patterning. Plant Cell 29:474–490
Shigematsu H, Iida K, Nakano M, Chaudhuri P, Iida H, Nagayama K (2014) Structural characterization of the mechanosensitive channel candidate MCA2 from Arabidopsis thaliana. PLoS ONE 9:e87724
Shih HW, Miller ND, Dai C, Spalding EP, Monshausen GB (2014) The receptor-like kinase FERONIA is required for mechanical signal transduction in Arabidopsis seedlings. Curr Biol 24:1887–1892
Stanković B, Davies E (1997) Intercellular communication in plants: electrical stimulation of proteinase inhibitor gene expression in tomato. Planta 202:402–406
Stanković B, Davies E (1998) The wound response in tomato involves rapid growth and electrical responses, systemically up-regulated transcription of proteinase inhibitor and calmodulin and down-regulated translation. Plant Cell Physiol 39:268–274
Steinhorst L, Kudla J (2013) Calcium and reactive oxygen species rule the waves of signaling. Plant Physiol 163:471–485
Suchyna TM, Johnson JH, Hamer K, Leykam JF, Gage DA, Clemo HF et al (2000) Identification of a peptide toxin from Grammostola spatulata spider venom that blocks cation-selective stretch-activated channels. J Gen Physiol 115:583–598
Taiz L (1984) Plant cell expansion: regulation of cell wall mechanical properties. Annu Rev Plant Physiol 35:585–657
Takemura K, Kamachi H, Kume A, Fujita T, Karahara I, Hanba YT (2017) A hypergravity environment increases chloroplast size, photosynthesis, and plant growth in the moss Physcomitrella patens. J Plant Res 130:181–192
Teng J, Loukin S, Anishkin A, Kung C (2015) The force-from-lipid (FFL) principle of mechanosensitivity, at large and in elements. Pflugers Arch 467:27–37
Toyota M, Furuichi T, Tatsumi H, Sokabe M (2007) Hypergravity stimulation induces changes in intracellular calcium concentration in Arabidopsis seedlings. Adv Space Res 39:1190–1197
Toyota M, Furuichi T, Tatsumi H, Sokabe M (2008a) Critical consideration on the relationship between auxin transport and calcium transients in gravity perception of Arabidopsis seedlings. Plant Signal Behav 3:521–524
Toyota M, Furuichi T, Tatsumi H, Sokabe M (2008b) Cytoplasmic calcium increases in response to changes in the gravity vector in hypocotyls and petioles of Arabidopsis seedlings. Plant Physiol 146:505–514
Toyota M, Furuichi T, Sokabe M, Tatsumi H (2013) Analyses of a gravistimulation-specific Ca2+ signature in Arabidopsis using parabolic flights. Plant Physiol 163:543–554
Toyota M, Gilroy S (2013) Gravitropism and mechanical signaling in plants. Am J Bot 100:111–125
Toyota M, Ikeda N, Tasaka M, Morita MT (2014) Centrifuge microscopy to analyze the sedimentary movements of amyloplasts. Bio-protocol 4:e1229
Veley KM, Marshburn S, Clure CE, Haswell ES (2012) Mechanosensitive channels protect plastids from hypoosmotic stress during normal plant growth. Curr Biol 22:408–413
Volkers L, Mechioukhi Y, Coste B (2015) Piezo channels: from structure to function. Pflugers Arch 467:95–99
Weinl S, Kudla J (2009) The CBL-CIPK Ca2+-decoding signaling network: function and perspectives. New Phytol 184:517–528
Wildon D, Thain J, Minchin P, Gubb I, Reilly A, Skipper Y et al (1992) Electrical signalling and systemic proteinase inhibitor induction in the wounded plant. Nature 360:62–65
Wilson ME, Jensen GS, Haswell ES (2011) Two mechanosensitive channel homologs influence division ring placement in Arabidopsis chloroplasts. Plant Cell 23:2939–2949
Wilson ME, Maksaev G, Haswell ES (2013) MscS-like mechanosensitive channels in plants and microbes. Biochemistry 52:5708–5722
Wormit A, Butt SM, Chairam I, McKenna JF, Nunes-Nesi A, Kjaer L et al (2012) Osmosensitive changes of carbohydrate metabolism in response to cellulose biosynthesis inhibition. Plant Physiol 159:105–117
Yamanaka T, Nakagawa Y, Mori K, Nakano M, Imamura T, Kataoka H et al (2010) MCA1 and MCA2 that mediate Ca2+ uptake have distinct and overlap** roles in Arabidopsis. Plant Physiol 152:1284–1296
Yang H, **e S, Wang L, **g S, Zhu X, Li X et al (2011) Identification of up-regulated genes in tea leaves under mild infestation of green leafhopper. Sci Hortic 130:476–481
Yuan F, Yang H, Xue Y, Kong D, Ye R, Li C et al (2014) OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 514:367–371
Zandomeni K, Schopfer P (1994) Mechanosensory microtubule reorientation in the epidermis of maize coleoptiles subjected to bending stress. Protoplasma 182:96–101
Zebelo SA, Maffei ME (2015) Role of early signalling events in plant-insect interactions. J Exp Bot 66:435–448
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Toyota, M., Furuichi, T., Iida, H. (2018). Molecular Mechanisms of Mechanosensing and Mechanotransduction. In: Geitmann, A., Gril, J. (eds) Plant Biomechanics. Springer, Cham. https://doi.org/10.1007/978-3-319-79099-2_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-79099-2_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-79098-5
Online ISBN: 978-3-319-79099-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)