Abstract
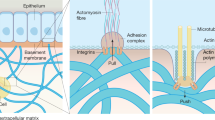
The implantation of materials into the body elicits a foreign body response (FBR) that includes formation of a fibrous capsule around the implanted material. The formation of the fibrous capsule has many similarities to fibrotic responses to other insults or stressors. A number of biochemical factors are known to promote a fibrotic response including growth factors, cytokines, and hormones. Much less is known regarding the role of biomechanical forces in tissue fibrosis. The biomechanical environment plays a fundamental role in embryonic development, tissue maintenance, and pathogenesis. Mechanical forces play particularly important roles in the regulation of connective tissues including not only bone and cartilage but also the interstitial tissues of most organs. In vivo studies have correlated changes in mechanical load to modulation of the extracellular matrix and have indicated that increased mechanical force contributes to the enhanced expression and deposition of extracellular matrix components or fibrosis. A variety of in vitro models have been utilized to evaluate the effects of mechanical force on extracellular matrix-producing cells. In general, application of mechanical stretch, fluid flow, and compression results in enhanced expression and deposition of extracellular matrix components. More recent studies have indicated that tissue rigidity also provides profibrotic signals to cells. This is particularly relevant to implants as the implanted material generally alters the local biomechanical environment, which may promote fibrosis or the formation of the fibrous capsule. The mechanisms whereby cells detect mechanical signals and transduce them into biochemical responses have received considerable attention. Cell surface receptors for extracellular matrix components and intracellular signaling pathways are instrumental in the mechanotransduction process. Understanding the effects of the biomechanical environment and the mechanisms, whereby mechanical forces are transduced into biochemical and molecular signals in the cell, will provide important insight into tissue fibrosis and fibrous capsule formation.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Beloussov LV, Grabovsky VI (2006) Morphomechanics: goals, basic experiments and models. Int J Dev Biol 50(2-3):81–92
Benjamin M, Hillen B (2003) Mechanical influences on cells, tissues and organs—‘mechanical morphogenesis’. Eur J Morphol 41(1):3–7
Farge E (2011) Mechanotransduction in development. Curr Top Dev Biol 95:243–265
Jones EA (2011) Mechanical factors in the development of the vascular bed. Respir Physiol Neurobiol 178(1):59–65
Bassett CA, Herrmann I (1961) Influence of oxygen concentration and mechanical factors on differentiation of connective tissues in vitro. Nature 190:460–461
Rodan GA, Mensi T, Harvey A (1975) A quantitative method for the application of compressive forces to bone in tissue culture. Calcif Tissue Res 18(2):125–131
Leung DY, Glagov S, Mathews MB (1976) Cyclic stretching stimulates synthesis of matrix components by arterial smooth muscle cells in vitro. Science 191(4226):475–477
Leung DY, Glagov S, Mathews MB (1977) A new in vitro system for studying cell response to mechanical stimulation. Different effects of cyclic stretching and agitation on smooth muscle cell biosynthesis. Exp Cell Res 109(2):285–298
Dzau VJ (1993) Local contractile and growth modulators in the myocardium. Clin Cardiol 16(5 Suppl 2):II5–II9
Samuel JL, Dubus I, Contard F et al (1990) Biological signals of cardiac hypertrophy. Eur Heart J 11 Suppl G:1–7
Lohler J, Timpl R, Jaenisch R (1984) Embryonic lethal mutation in mouse collagen I gene causes rupture of blood vessels and is associated with erythropoietic and mesenchymal cell death. Cell 38(2):597–607
Brenner DA, Kisseleva T, Scholten D et al (2012) Origin of myofibroblasts in liver fibrosis. Fibrogenesis Tissue Repair 5(Suppl 1):S17
Hinz B (2012) Mechanical aspects of lung fibrosis: a spotlight on the myofibroblast. Proc Am Thorac Soc 9(3):137–147
Hinz B, Phan SH, Thannickal VJ et al (2012) Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol 180(4):1340–1355
Park S, Park M, Kim BH et al (2015) Acute suppression of TGF-ss with local, sustained release of tranilast against the formation of fibrous capsules around silicone implants. J Control Release 200:125–137
Wolfram D, Rainer C, Niederegger H et al (2004) Cellular and molecular composition of fibrous capsules formed around silicone breast implants with special focus on local immune reactions. J Autoimmun 23(1):81–91
Bowen T, Jenkins RH, Fraser DJ (2013) MicroRNAs, transforming growth factor beta-1, and tissue fibrosis. J Pathol 229(2):274–285
Mammoto T, Ingber DE (2010) Mechanical control of tissue and organ development. Development 137(9):1407–1420
Tomeno W, Yoneda M, Imajo K et al (2013) Evaluation of the Liver Fibrosis Index calculated by using real-time tissue elastography for the non-invasive assessment of liver fibrosis in chronic liver diseases. Hepatol Res 43(7):735–742
Yin MF, Lian LH, Piao DM et al (2007) Tetrandrine stimulates the apoptosis of hepatic stellate cells and ameliorates development of fibrosis in a thioacetamide rat model. World J Gastroenterol 13(8):1214–1220
Lam WA, Cao L, Umesh V et al (2010) Extracellular matrix rigidity modulates neuroblastoma cell differentiation and N-myc expression. Mol Cancer 9:35
Paszek MJ, Zahir N, Johnson KR et al (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8(3):241–254
Pathak A, Kumar S (2012) Independent regulation of tumor cell migration by matrix stiffness and confinement. Proc Natl Acad Sci U S A 109(26):10334–10339
Schaller MD, Borgman CA, Cobb BS et al (1992) pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A 89(11):5192–5196
Schedin P, Keely PJ (2011) Mammary gland ECM remodeling, stiffness, and mechanosignaling in normal development and tumor progression. Cold Spring Harb Perspect Biol 3(1):a003228
Ulrich TA, de Juan Pardo EM, Kumar S (2009) The mechanical rigidity of the extracellular matrix regulates the structure, motility, and proliferation of glioma cells. Cancer Res 69(10):4167–4174
Choi TY, Ahmadi N, Sourayanezhad S et al (2013) Relation of vascular stiffness with epicardial and pericardial adipose tissues, and coronary atherosclerosis. Atherosclerosis 229(1):118–123
Wells RG (2013) Tissue mechanics and fibrosis. Biochim Biophys Acta 1832(7):884–890
Ho YY, Lagares D, Tager AM et al (2014) Fibrosis—a lethal component of systemic sclerosis. Nat Rev Rheumatol 10(7):390–402
Clark RA, Ashcroft GS, Spencer MJ et al (1996) Re-epithelialization of normal human excisional wounds is associated with a switch from alpha v beta 5 to alpha v beta 6 integrins. Br J Dermatol 135(1):46–51
Hinz B (2009) Tissue stiffness, latent TGF-beta1 activation, and mechanical signal transduction: implications for the pathogenesis and treatment of fibrosis. Curr Rheumatol Rep 11(2):120–126
Wang HB, Dembo M, Wang YL (2000) Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am J Physiol Cell Physiol 279(5):C1345–C1350
Yeung T, Georges PC, Flanagan LA et al (2005) Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton 60(1):24–34
Peyton SR, Putnam AJ (2005) Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J Cell Physiol 204(1):198–209
Engler AJ, Rehfeldt F, Sen S et al (2007) Microtissue elasticity: measurements by atomic force microscopy and its influence on cell differentiation. Methods Cell Biol 83:521–545
Georges PC, Hui JJ, Gombos Z et al (2007) Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol 293(6):G1147–G1154
Mauch C, Adelmann-Grill B, Hatamochi A et al (1989) Collagenase gene expression in fibroblasts is regulated by a three-dimensional contact with collagen. FEBS Lett 250(2):301–305
Johnson LA, Rodansky ES, Sauder KL et al (2013) Matrix stiffness corresponding to strictured bowel induces a fibrogenic response in human colonic fibroblasts. Inflamm Bowel Dis 19(5):891–903
Liu F, Mih JD, Shea BS et al (2010) Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J Cell Biol 190(4):693–706
Arora PD, Narani N, McCulloch CA (1999) The compliance of collagen gels regulates transforming growth factor-beta induction of alpha-smooth muscle actin in fibroblasts. Am J Pathol 154(3):871–882
Galie PA, Westfall MV, Stegemann JP (2011) Reduced serum content and increased matrix stiffness promote the cardiac myofibroblast transition in 3D collagen matrices. Cardiovasc Pathol 20(6):325–333
Huang X, Yang N, Fiore VF et al (2012) Matrix stiffness-induced myofibroblast differentiation is mediated by intrinsic mechanotransduction. Am J Respir Cell Mol Biol 47(3):340–348
Olsen AL, Bloomer SA, Chan EP et al (2011) Hepatic stellate cells require a stiff environment for myofibroblastic differentiation. Am J Physiol Gastrointest Liver Physiol 301(1):G110–G118
Shi Y, Dong Y, Duan Y et al (2013) Substrate stiffness influences TGF-beta1-induced differentiation of bronchial fibroblasts into myofibroblasts in airway remodeling. Mol Med Rep 7(2):419–424
Friedman SL (2008) Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 88(1):125–172
Friedman SL, Roll FJ, Boyles J et al (1989) Maintenance of differentiated phenotype of cultured rat hepatic lipocytes by basement membrane matrix. J Biol Chem 264(18):10756–10762
Gaca MD, Zhou X, Issa R et al (2003) Basement membrane-like matrix inhibits proliferation and collagen synthesis by activated rat hepatic stellate cells: evidence for matrix-dependent deactivation of stellate cells. Matrix Biol 22(3):229–239
Wang H, Haeger SM, Kloxin AM et al (2012) Redirecting valvular myofibroblasts into dormant fibroblasts through light-mediated reduction in substrate modulus. PLoS One 7(7), e39969
Balestrini JL, Chaudhry S, Sarrazy V et al (2012) The mechanical memory of lung myofibroblasts. Integr Biol (Camb) 4(4):410–421
Wilson CG, Stone JW, Fowlkes V et al (2011) Age-dependent expression of collagen receptors and deformation of type I collagen substrates by rat cardiac fibroblasts. Microsc Microanal 17(4):555–562
Wang S, Cukierman E, Swaim WD et al (1999) Extracellular matrix protein-induced changes in human salivary epithelial cell organization and proliferation on a model biological substratum. Biomaterials 20(11):1043–1049
Zhang YH, Zhao CQ, Jiang LS et al (2011) Substrate stiffness regulates apoptosis and the mRNA expression of extracellular matrix regulatory genes in the rat annular cells. Matrix Biol 30(2):135–144
Vandenburgh HH (1982) Dynamic mechanical orientation of skeletal myofibers in vitro. Dev Biol 93(2):438–443
Vandenburgh H, Kaufman S (1979) In vitro model for stretch-induced hypertrophy of skeletal muscle. Science 203(4377):265–268
Weber KT, Janicki JS, Shroff SG et al (1988) Collagen remodeling of the pressure-overloaded, hypertrophied nonhuman primate myocardium. Circ Res 62(4):757–765
Jalil JE, Doering CW, Janicki JS et al (1989) Fibrillar collagen and myocardial stiffness in the intact hypertrophied rat left ventricle. Circ Res 64(6):1041–1050
Kollros PR, Bates SR, Mathews MB et al (1987) Cyclic AMP inhibits increased collagen production by cyclically stretched smooth muscle cells. Lab Invest 56(4):410–417
Butt RP, Bishop JE (1997) Mechanical load enhances the stimulatory effect of serum growth factors on cardiac fibroblast procollagen synthesis. J Mol Cell Cardiol 29(4):1141–1151
Carver W, Nagpal ML, Nachtigal M et al (1991) Collagen expression in mechanically stimulated cardiac fibroblasts. Circ Res 69(1):116–122
Lee AA, Delhaas T, Waldman LK et al (1996) An equibiaxial strain system for cultured cells. Am J Physiol 271(4 Pt 1):C1400–C1408
Wang Z, Kuang R, Xu Q et al (2015) Reaction of human fibroblasts from different sites to the mechanical stress. Zhongguo **u Fu Chong Jian Wai Ke Za Zhi 29(4):467–471
Auluck A, Mudera V, Hunt NP et al (2005) A three-dimensional in vitro model system to study the adaptation of craniofacial skeletal muscle following mechanostimulation. Eur J Oral Sci 113(3):218–224
Birla RK, Huang YC, Dennis RG (2007) Development of a novel bioreactor for the mechanical loading of tissue-engineered heart muscle. Tissue Eng 13(9):2239–2248
Masoumi N, Howell MC, Johnson KL et al (2014) Design and testing of a cyclic stretch and flexure bioreactor for evaluating engineered heart valve tissues based on poly(glycerol sebacate) scaffolds. Proc Inst Mech Eng H 228(6):576–586
Tokuyama E, Nagai Y, Takahashi K et al (2015) Mechanical stretch on human skin equivalents increases the epidermal thickness and develops the basement membrane. PLoS One 10(11), e0141989
Imsirovic J, Derricks K, Buczek-Thomas JA et al (2013) A novel device to stretch multiple tissue samples with variable patterns: application for mRNA regulation in tissue-engineered constructs. Biomatter 3(3):pii: e24650
Obi S, Yamamoto K, Ando J (2014) Effects of shear stress on endothelial progenitor cells. J Biomed Nanotechnol 10(10):2586–2597
Dunn J, Simmons R, Thabet S et al (2015) The role of epigenetics in the endothelial cell shear stress response and atherosclerosis. Int J Biochem Cell Biol 67:167–176
Rodriguez I, Gonzalez M (2014) Physiological mechanisms of vascular response induced by shear stress and effect of exercise in systemic and placental circulation. Front Pharmacol 5:209
Sedmera D, Pexieder T, Rychterova V et al (1999) Remodeling of chick embryonic ventricular myoarchitecture under experimentally changed loading conditions. Anat Rec 254(2):238–252
Hove JR, Koster RW, Forouhar AS et al (2003) Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421(6919):172–177
Tan H, Biechler S, Junor L et al (2013) Fluid flow forces and rhoA regulate fibrous development of the atrioventricular valves. Dev Biol 374(2):345–356
Egorova AD, Khedoe PP, Goumans MJ et al (2011) Lack of primary cilia primes shear-induced endothelial-to-mesenchymal transition. Circ Res 108(9):1093–1101
Misra S, Fu AA, Puggioni A et al (2008) Increased shear stress with upregulation of VEGF-A and its receptors and MMP-2, MMP-9, and TIMP-1 in venous stenosis of hemodialysis grafts. Am J Physiol Heart Circ Physiol 294(5):H2219–H2230
Markwald RR, Fitzharris TP, Manasek FJ (1977) Structural development of endocardial cushions. Am J Anat 148(1):85–119
Zeisberg EM, Tarnavski O, Zeisberg M et al (2007) Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med 13(8):952–961
Zeisberg EM, Potenta SE, Sugimoto H et al (2008) Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol 19(12):2282–2287
Moonen JR, Lee ES, Schmidt M et al (2015) Endothelial-to-mesenchymal transition contributes to fibro-proliferative vascular disease and is modulated by fluid shear stress. Cardiovasc Res 108(3):377–386
Grabias BM, Konstantopoulos K (2014) The physical basis of renal fibrosis: effects of altered hydrodynamic forces on kidney homeostasis. Am J Physiol Renal Physiol 306(5):F473–F485
Chao YH, Tsuang YH, Sun JS et al (2012) Centrifugal force induces human ligamentum flavum fibroblasts inflammation through activation of JNK and p38 pathways. Connect Tissue Res 53(5):422–429
Nogueira AV, Nokhbehsaim M, Eick S et al (2014) Biomechanical loading modulates proinflammatory and bone resorptive mediators in bacterial-stimulated PDL cells. Mediators Inflamm 2014:425421
Jacobs C, Walter C, Ziebart T et al (2014) Induction of IL-6 and MMP-8 in human periodontal fibroblasts by static tensile strain. Clin Oral Investig 18(3):901–908
D’Angelo E, Koutsoukou A, Della Valle P et al (2008) Cytokine release, small airway injury, and parenchymal damage during mechanical ventilation in normal open-chest rats. J Appl Physiol (1985) 104(1):41–49
Plataki M, Hubmayr RD (2010) The physical basis of ventilator-induced lung injury. Expert Rev Respir Med 4(3):373–385
Li G, Luna C, Qiu J et al (2010) Modulation of inflammatory markers by miR-146a during replicative senescence in trabecular meshwork cells. Invest Ophthalmol Vis Sci 51(6):2976–2985
Huang Y, Crawford M, Higuita-Castro N et al (2012) miR-146a regulates mechanotransduction and pressure-induced inflammation in small airway epithelium. FASEB J 26(8):3351–3364
Hartupee J, Mann DL (2016) Role of inflammatory cells in fibroblast activation. J Mol Cell Cardiol 93:143–148
Madjene LC, Pons M, Danelli L et al (2015) Mast cells in renal inflammation and fibrosis: lessons learnt from animal studies. Mol Immunol 63(1):86–93
Overed-Sayer C, Rapley L, Mustelin T et al (2013) Are mast cells instrumental for fibrotic diseases? Front Pharmacol 4:174
Fowlkes V, Wilson CG, Carver W et al (2013) Mechanical loading promotes mast cell degranulation via RGD-integrin dependent pathways. J Biomech 46(4):788–795
Komiyama H, Miyake K, Asai K et al (2014) Cyclical mechanical stretch enhances degranulation and IL-4 secretion in RBL-2H3 mast cells. Cell Biochem Funct 32(1):70–76
DuFort CC, Paszek MJ, Weaver VM (2011) Balancing forces: architectural control of mechanotransduction. Nat Rev Mol Cell Biol 12(5):308–319
Zhang H, Labouesse M (2012) Signalling through mechanical inputs: a coordinated process. J Cell Sci 125(Pt 13):3039–3049
MacKenna DA, Dolfi F, Vuori K et al (1998) Extracellular signal-regulated kinase and c-Jun NH2-terminal kinase activation by mechanical stretch is integrin-dependent and matrix-specific in rat cardiac fibroblasts. J Clin Invest 101(2):301–310
Buck CA, Horwitz AF (1987) Cell surface receptors for extracellular matrix molecules. Annu Rev Cell Biol 3:179–205
Humphries MJ, Yasuda Y, Olden K et al (1988) The cell interaction sites of fibronectin in tumour metastasis. Ciba Found Symp 141:75–93
Atance J, Yost MJ, Carver W (2004) Influence of the extracellular matrix on the regulation of cardiac fibroblast behavior by mechanical stretch. J Cell Physiol 200(3):377–386
Roca-Cusachs P, Iskratsch T, Sheetz MP (2012) Finding the weakest link: exploring integrin-mediated mechanical molecular pathways. J Cell Sci 125(Pt 13):3025–3038
Zaidel-Bar R, Itzkovitz S, Ma’ayan A et al (2007) Functional atlas of the integrin adhesome. Nat Cell Biol 9(8):858–867
Pasapera AM, Schneider IC, Rericha E et al (2010) Myosin II activity regulates vinculin recruitment to focal adhesions through FAK-mediated paxillin phosphorylation. J Cell Biol 188(6):877–890
Ingber DE (1991) Control of capillary growth and differentiation by extracellular matrix. Use of a tensegrity (tensional integrity) mechanism for signal processing. Chest 99(3 Suppl):34S–40S
Ingber DE (1997) Integrins, tensegrity, and mechanotransduction. Gravit Space Biol Bull 10(2):49–55
Burridge K, Mangeat P (1984) An interaction between vinculin and talin. Nature 308(5961):744–746
Critchley DR (2009) Biochemical and structural properties of the integrin-associated cytoskeletal protein talin. Annu Rev Biophys 38:235–254
Weiner TM, Liu ET, Craven RJ et al (1993) Expression of focal adhesion kinase gene and invasive cancer. Lancet 342(8878):1024–1025
Chen HC, Appeddu PA, Isoda H et al (1996) Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J Biol Chem 271(42):26329–26334
Michael KE, Dumbauld DW, Burns KL et al (2009) Focal adhesion kinase modulates cell adhesion strengthening via integrin activation. Mol Biol Cell 20(9):2508–2519
Mammoto A, Mammoto T, Ingber DE (2012) Mechanosensitive mechanisms in transcriptional regulation. J Cell Sci 125(Pt 13):3061–3073
Carter DR, Beaupre GS, Giori NJ et al (1998) Mechanobiology of skeletal regeneration. Clin Orthop Relat Res (355 Suppl):S41–S55
Zheng W, Christensen LP, Tomanek RJ (2008) Differential effects of cyclic and static stretch on coronary microvascular endothelial cell receptors and vasculogenic/angiogenic responses. Am J Physiol Heart Circ Physiol 295(2):H794–H800
Lehoux S, Esposito B, Merval R et al (2005) Differential regulation of vascular focal adhesion kinase by steady stretch and pulsatility. Circulation 111(5):643–649
Discher DE, Mooney DJ, Zandstra PW (2009) Growth factors, matrices, and forces combine and control stem cells. Science 324(5935):1673–1677
Bishop JE, Butt R, Dawes K et al (1998) Mechanical load enhances the stimulatory effect of PDGF on pulmonary artery fibroblast procollagen synthesis. Chest 114(1 Suppl):25S
Wipff PJ, Rifkin DB, Meister JJ et al (2007) Myofibroblast contraction activates latent TGF-beta1 from the extracellular matrix. J Cell Biol 179(6):1311–1323
Tomasek JJ, Gabbiani G, Hinz B et al (2002) Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3(5):349–363
Goffin JM, Pittet P, Csucs G et al (2006) Focal adhesion size controls tension-dependent recruitment of alpha-smooth muscle actin to stress fibers. J Cell Biol 172(2):259–268
Wipff PJ, Hinz B (2008) Integrins and the activation of latent transforming growth factor beta1—an intimate relationship. Eur J Cell Biol 87(8-9):601–615
Sarrazy V, Koehler A, Chow ML et al (2014) Integrins alphavbeta5 and alphavbeta3 promote latent TGF-beta1 activation by human cardiac fibroblast contraction. Cardiovasc Res 102(3):407–417
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Carver, W., Esch, A.M., Fowlkes, V., Goldsmith, E.C. (2017). The Biomechanical Environment and Impact on Tissue Fibrosis. In: Corradetti, B. (eds) The Immune Response to Implanted Materials and Devices. Springer, Cham. https://doi.org/10.1007/978-3-319-45433-7_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-45433-7_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-45431-3
Online ISBN: 978-3-319-45433-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)