Abstract
Continence can be defined as the ability to retain flatus, liquid, or solid stools during normal daily life, including while physically exercising, coughing, sneezing, and changing position. It can be considered as a somatovisceral reflex, and it involves complex coordinated activity between several muscle groups—including the sphincter complex, ligaments and fascia of the pelvic floor—and rectal compliance, stool consistency and volume, and cognitive function. Consequently, an abnormality in any of these factors may result in fecal incontinence (FI). Moreover, considering the heterogeneity of the problem, the incidence and prevalence of FI are difficult to establish because they depend on the type and frequency of incontinence, age, and gender. The aim of this chapter is to illustrate the anatomy and physiology of continence as well as the possible pathophysiological reasons that lead to the development of FI.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- Fecal incontinence
- Anal canal
- Sphincter complex
- Post-obstetric injury
- Anorectal surgery
- Rectal sensation
- Functional disorders
1 Introduction and Epidemiology
Continence can be defined as the ability to retain flatus, liquid, or solid stools during normal daily life, including while physically exercising, coughing, sneezing, and changing position [1].
The anal canal is normally closed at rest and during sleep due to the steady activity of the internal anal sphincter (IAS) supported by the tonic activity of the external anal sphincter (EAS) and puborectalis.
The integrity of the continence and defecation mechanism is a multifactorial process that involves somatic and visceral functions and allows postponing defecation when necessary and avoiding the uncontrolled passage of feces or gas, causing patients to feel embarrassed with a negative impact on lifestyle, work, and interpersonal relationships [2].
Considering the heterogeneity of the problem, the incidence and prevalence of fecal incontinence (FI) are difficult to establish because they depend on the type and frequency of incontinence, age, and gender (Table 2.1) [3]. In fact, if the leading cause in women is post-obstetric injury, in men and the elderly, other factors such as anorectal surgery and diabetes mellitus must be considered [4, 5]. Moreover, the use of many terms to define FI has generated confusion and favored this trend.
Several authors have tried to quantify this phenomenon without achieving a definitive percentage [6,7,8]. According to Sharma et al. [6], the prevalence of FI ranges between 1.4% and 19.5%, whereas in the systematic review by Ng et al. [7] it has a median prevalence of 7.7%, without any difference between genders, but with a greater percentage in people older than 90 (15.9% vs. 5.7%) compared with people 15–34 years old.
The higher prevalence among the elderly may be due to the physiological effects of aging on continence, such as impaired rectal sensation or dysfunction of both the IAS and EAS, which become thicker [9], as well as polypharmacy. In fact, the abuse of laxatives taken to avoid fecal impaction or constipation can exacerbate the condition. In this context, the highest prevalence currently recorded is among nursing home residents, where involvement reaches up to 50% [10].
The difficulty in establishing the extent of FI could be due to embarrassment in reporting the symptoms, as occurs with other proctological diseases [11]. Brown et al. [12], in an internet-based questionnaire study of 5817 women, showed that one-fifth of women over 45 in the USA suffer from at least one episode of FI per year. A lack of knowledge and awareness of the problem as well as economic status represent further important barriers to patients’ interaction with the healthcare system.
2 Anatomy and Physiology of Continence
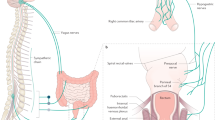
The continence process can be defined as a somatovisceral reflex and involves complex coordinated activity between several muscle groups – including the sphincter complex (Fig. 2.1) [3], ligaments and fascia of the pelvic floor—and rectal compliance, stool consistency and volume, and cognitive function. Thus, an abnormality in any of these factors may result in FI. In fact, up to 80% of patients with anal incontinence have more than one pathological abnormality in anorectal physiology.
Structure of the anorectum. The internal anal sphincter muscle provides between 70% and 85% of resting sphincter pressure. The anorectal angle, approximately 90 degrees at rest, becomes more obtuse during defecation. (Reproduced from [3] with permission from Elsevier)
The IAS (Fig. 2.2) [13] represents the continuation of the inner circular muscle layer of the rectum [14]. This smooth muscle under the control of the involuntary nervous system (sympathetic and parasympathetic) contributes to 80% of the resting anal pressure—normally 50–70 mmHg—and is the main barrier to the involuntary passage of gas and feces. The remaining 15–20% of resting anal pressure is regulated by the EAS, puborectalis, and hemorrhoids. All three contribute to the high-pressure zone, which is appreciated along the entire length of the anal canal and has 30% higher than found in the rectum. Interestingly, according to Penninckx et al. [15], the resting anal pressure is generated by the myogenic tone of the IAS (10%), by nerve-induced activity (45%), by the EAS (35%), and by the hemorrhoids (15%).
(a) Endoanal scan demonstrating the U-shaped puborectalis muscle, which attaches to the pubic rami anteriorly. (b) Endoanal scan demonstrating the internal anal sphincter (white arrow) and the external anal sphincter (black arrow). (c) Three-dimensional endoanal ultrasound demonstrating the circumference/width as well as length of the anal sphincter defect. (Reproduced from [13] with permission from British Institute of Radiology)
The length of the IAS differs between genders and is smaller in women (2.0–3.0 cm vs. 2.5–3.5 cm), while its thickness varies between 2 and 4 mm. The IAS has a resting tone with cyclic variations, or rather short and ultrashort waves (1.5–3 cycles per minute). These waves are extremely important for reflex sampling. In fact, an intermittent relaxation of the IAS, lasting about 10–20 s, occurs approximately seven times per hour [16, 17] and allows rectal contents to come into contact with the sensitive mucosa of the anal canal.
The EAS (Fig. 2.2) [13] is a striated muscle that receives innervation from the pudendal nerve (S2, S3, S4), and its inhibition allows defecation [18]. It has a thickness of 4 mm and length of about 2.7 cm, although it is shorter anteriorly in women (1.5 cm), and it has three components: the subcutaneous, superficial, and deep anorectal elements. The EAS is responsible for the guarding reflex, a low spinal reflex, which contracts for 20–30 s after fecal contents arrive in the rectal ampulla.
The levator ani (Fig. 2.2) [13] has a pelvic floor support function and consists of three bundles: the pubococcygeus anteriorly, the iliococcygeus posteriorly, and the puborectalis inferiorly. The most important component for continence is the puborectalis muscle (PRM), which is a U-shaped loop muscle that forms a sling around the rectum, with an anorectal angle of approximately 90° that is at the basis of the flap-valve theory proposed by Parks [19], to which the three portions of the EAS also contribute. During normal defecation, relaxation of the PRM causes the anorectal angle to become more obtuse and widen (>130°), facilitating the passage of the rectal content.
Recently, Broens et al. [20] introduced the concept of the “puborectalis continence reflex”, demonstrating that the PRM has also an involuntary contraction that is significantly stronger than the voluntary one with an equally increased pressure zone. This could confirm the role of the PRM in supporting dysfunctions of the other sphincter muscles in patients with impaired continence but who have not developed FI.
The conjoined longitudinal muscle, interposed between the IAS and EAS, consists of smooth muscle together with striated fibers from the levator ani, and its role is controversial. It has a thickness of about 2.5 mm and seems involved in the shortening and widening of the anal canal. Shafik originally defined it as an “evertor ani muscle” [21] for its apparent action of eversion of the anal orifice. In addition, some studies have recently suggested its role in supporting continence after lateral internal sphincterotomy [22].
In addition to the muscular component, several other factors contribute to anal continence. The recto-anal inhibitory reflex (RAIR), also known as relaxation of the IAS, is certainly one of the best-established mechanisms linked to defecation and among the first to be activated.
It is regulated by the enteric nervous system, which is why it is absent in Hirschsprung’s disease, but it is still present after rectum or anus denervation or recovers with time [23].
The distension of the rectal wall caused by the arrival of the intestinal contents, made possible by the action of another important functional apparatus—the “rectosigmoid sphincter” [24, 25]—thanks to colonic high-amplitude-propagated contractions, stimulates the multiple pressure receptors, allowing contact with the sensitive area of the anal canal. In this area, the stool contents are in contact with specialized sensory organs such as the Krause end-bulbs, Golgi-Mazzoni’s bodies and genital corpuscles, the sparse Meissner’s corpuscles, and Pacinian corpuscles [26, 27].
At this point, continence is maintained by the EAS (rectoanal excitatory reflex [RAER]) that may avoid anal leakage, and, to occur, intra-abdominal pressure should increase, with consequent relaxation of the PRM, widening of the anorectal angle, and rectification of the rectum. The reflex contraction of the EAS with the concomitant contraction of the PRM to restore the anorectal angle ends the process. If defecation is deemed inappropriate, it can be deferred by the voluntary contraction of both EAS and PRM [28]. In particular, the PRM induces closure of the pelvic diaphragm by generating horizontal forces with a consequent decrease of the anorectal angle. Both RAIR and RAER make up the sampling reflex, which has the fundamental function of advancing a part of the rectal content in the upper anal canal without causing an episode of incontinence.
Under physiological conditions, the rectum can passively undergo dilation without pressure changes. At a volume of approximately 200 mL, a sensation of urgency is perceived, with a maximum tolerated volume ranging from 300 to 500 mL.
Pathological alterations of the rectum, such as inflammatory bowel disease, in which rectal compliance decreases, or Hirschsprung’s disease, in which compliance is increased, favor altered continence.
Hemorrhoids are vascular cushions contributing to almost 15% of the anal resting pressure, and their importance has been seen in transient or permanent soiling episodes that can occur after excisional hemorrhoidectomy [29].
The volume and consistency of feces are essential, especially in elderly patients or patients with an already impaired continence, such as after low anterior resection [5].
Lastly, it is not only the peripheral nervous system with the sympathetic (L1–L3) and parasympathetic (S2–S4) components that is involved, but several studies have highlighted the role of the central nervous system. In fact, the sensation of rectal filling or urgency has been shown to be associated with areas such as the insula, thalamus, secondary somatosensory cortex, or anterior cingulate gyrus [30], whereas Brodmann area 4, the primary motor cortex, seems involved in anal and rectal responses [31].
Onuf’s nucleus, positioned at the level of the ventral horn gray matter of S2–S4, is in communication with the upper motor neurons responsible for voluntary sphincter complex contraction, usually located in the parasagittal motor cortex [32].
3 Pathophysiology of Fecal Incontinence
Given the complexity of the continence mechanism, several target areas of injuries can lead to the development of FI. Moreover, it appears that more than 80% of patients with FI have more than one alteration of the continence mechanism [33].
Depending on the type of muscle injury, FI can have different manifestations. Urge incontinence, or the loss of stools despite a voluntary attempt to avoid it, is caused by a lesion of the EAS. When the IAS is involved, it is referred to as passive incontinence, with involuntary loss of liquid or stool without awareness. Both conditions are very common after obstetric trauma, which can also be caused by the EAS, IAS, and the pudendal nerve being stretched, compressed, or suffering an ischemic injury (Fig. 2.2) [13]. The pudendal nerve, certainly the most studied but not the only nerve involved in the continence mechanism, can be damaged during childbirth at the exit from Alcock’s canal, where its course is predominantly fixed on the pelvic sidewall [34].
Episiotomy appears to be related to damage to the sphincter complex, even if some studies show conflicting opinions [35, 36]. In particular, midline posterior episiotomy has been correlated with a higher incidence of sphincter trauma [37].
According to Dudding et al. [38], episiotomy and instrumental delivery, fetal occipito-posterior presentation, a prolonged second stage of labor, and birth weight greater than 4 kg are risk factors for injury. Interestingly, most women develop FI after menopause, maybe due to the deterioration of anorectal function with aging or withdrawal of hormonal input. Consequently, sphincter damage represents the first step for develo** FI with the overlap of other factors [39].
Urge incontinence can also be secondary to anorectal surgery (hemorrhoidectomy, sphincterotomy, surgery for fistulas), albeit more frequently in men, with the contemporary loss of the sampling reflex.
Another component of the sphincter complex that can be damaged is the PRM, often following an accidental trauma, perineal descent, or aging, with loss of functionality of the anorectal angle.
Often, FI and constipation may coexist, and in this case we define incontinence as fecal seepage or the involuntary post-defecation loss of stool [40]. Fecal seepage results from an impaired rectal sensation plus an inappropriate increase of anal sphincter pressure with the contraction of the EAS after excessive straining.
In men, the causes of FI are less defined. Radical prostatectomy with consequent radiotherapy can lead to sphincter fibrosis, myenteric plexus degeneration, and a reduction of the functionality of the EAS, which becomes thicker with reduced rectal compliance, especially in the elderly. The same phenomenon occurs during radiotherapy for anal or rectal cancer.
When the reservoir function of the rectum is no longer optimal, increased intrarectal pressure can cause FI. This scenario can occur in patients with inflammatory bowel disease, radiation proctitis, hysterectomy, rectal cancer, or spinal cord injury.
Among the functional mechanisms, the loss of anorectal sensation is certainly the most frequent.
In fact, an impaired anorectal sensation, which often occurs in children and the elderly, can lead to fecal impaction and a consequent fecal overflow. Some of the most common causes are represented by neuropathies such as multiple sclerosis, diabetes mellitus, and spinal cord injury or by inadvertent injury during colorectal surgery. Central nervous system diseases, such as Parkinson’s disease, can alter cognitive functions, leading to pathological toilet habits due to the inability to carry out daily activities.
Hellström et al. [41] highlighted the influence of dementia on anal continence in a random sample of 485 subjects selected from the 85-year-old inhabitants of Gothenburg, reporting its presence in 34.8% of demented subjects and 6.7% of non-demented subjects.
Furthermore, fecal impaction, common in patients with obstructed bowel syndrome or pelvic floor dyssynergy, can result in stool leaks that bypass the impaction owing to persistent relaxation of the IAS. Overflow incontinence can also be present in patients with congenital malformations [42].
Another very common condition is soiling, which often occurs after anorectal surgery or in patients with obstructed defecation. Up to 63% of patients with grade II–IV hemorrhoidal disease or rectal prolapse may have soiling [43].
Finally, the change in stool volume and consistency due to inflammatory bowel disease, drugs (such as laxatives in the elderly), food intolerance, or metabolic disorders can cause diarrhea and urgency, fecal impaction, and malabsorption. In the case of diarrhea, increased activity of high-amplitude-propagated contractions can cause overwhelm of the reservoir capacity of the rectum [44, 45]. These motor complexes, extremely represented at the level of the rectosigmoid, are significantly increased in patients with urge incontinence [46].
References
Saldana Ruiz N, Kaiser AM. Fecal incontinence – Challenges and solutions. World J Gastroenterol. 2017;23(1):11–24.
Wald A. Clinical practice. Fecal incontinence in adults. N Engl J Med. 2007;356(16):1648–55.
Rao SS. Pathophysiology of adult fecal incontinence. Gastroenterology. 2004;126(1 Suppl 1):S14–22.
Nelson RL. Epidemiology of fecal incontinence. Gastroenterology. 2004;126(1 Suppl 1):S3–7.
Pucciani F. Post-surgical fecal incontinence. Updat Surg. 2018;70(4):477–84.
Sharma A, Yuan L, Marshall RJ, et al. Systematic review of the prevalence of faecal incontinence. Br J Surg. 2016;103(12):1589–97.
Ng KS, Sivakumaran Y, Nassar N, Gladman MA. Fecal incontinence: community prevalence and associated factors – a systematic review. Dis Colon Rectum. 2015;58(12):1194–209.
Macmillan AK, Merrie AEH, Marshall RJ, Parry BR. The prevalence of fecal incontinence in community-dwelling adults: a systematic review of the literature. Dis Colon Rectum. 2004;47(8):1341–9.
Lewicky-Gaupp C, Hamilton Q, Ashton-Miller J, et al. Anal sphincter structure and function relationships in aging and fecal incontinence. Am J Obstet Gynecol. 2009;200(5):559.e1–5.
Gorina Y, Schappert S, Bercovitz A, et al. Prevalence of incontinence among older Americans. Vital Health Stat 3. 2014;36:1–33.
Gallo G, Sacco R, Sammarco G. Epidemiology of hemorrhoidal disease. In: Ratto C, Parello A, Litta F, editors. Hemorrhoids coloproctology. Cham: Springer; 2018. p. 3–7.
Brown HW, Rogers RG, Wise ME. Barriers to seeking care for accidental bowel leakage: a qualitative study. Int Urogynecol J. 2017;28(4):543–51.
Abdool Z, Sultan AH, Thakar R. Ultrasound imaging of the anal sphincter complex: a review. Br J Radiol. 2012;85(1015):865–75.
Golia Pernicka JS, Sheedy SP, Ernst RD, et al. MR staging of anal cancer: what the radiologist needs to know. Abdom Radiol (NY). 2019;44(11):3726–39.
Penninckx F, Lestar B, Kerremans R. The internal anal sphincter: mechanisms of control and its role in maintaining anal continence. Baillieres Clin Gastroenterol. 1992;6(1):193–214.
Read NW, Haynes WG, Bartolo DC, et al. Use of anorectal manometry during rectal infusion of saline to investigate function in incontinent patients. Gastroenterology. 1983;85:105–13.
Miller R, Lewis GT, Bartolo DC, et al. Sensory discrimination and dynamic activity in the anorectum evidence of a new ambulatory technique. Br J Surg. 1988;75:1003–7.
Barleben A, Mills S. Anorectal anatomy and physiology. Surg Clin North Am. 2010;90(1):1–15.
Parks AG, Porter NH, Hardcastle J. The syndrome of the descending perineum. Proc R Soc Med. 1966;59:477–82.
Broens PMA, Jonker JE, Trzpis M. The puborectal continence reflex: a new regulatory mechanism controlling fecal continence. Int J Colorectal Dis. 2018;33(5):627–33.
Shafik A. A new concept of the anatomy of the anal sphincter mechanism and the physiology of defecation. III. The longitudinal anal muscle: anatomy and role in anal sphincter mechanism. Investig Urol. 1976;13:271–7.
Perry WB, Dykes SL, Buie WD, Rafferty JF. Standards Practice Task Force of the American Society of Colon and Rectal Surgeons. Practice parameters for the management of anal fissures (3rd revision). Dis Colon Rectum. 2010;53(8):1110–5.
Lubowski DZ, Nicholls RJ, Swash M, Jordan MJ. Neural control of internal anal sphincter function. Br J Surg. 1987;74(8):668–70.
Wadhwa RP, Mistry FP, Bhatia SJ, et al. Existence of a high pressure zone at the rectosigmoid junction in normal Indian men. Dis Colon Rectum. 1996;39:1122–5.
Ballantyne GH. Rectosigmoid sphincter of O’Beirne. Dis Colon Rectum. 1986;29:525–31.
Duthie HL, Gaims FW. Sensory nerve-endings and sensation in the anal region of man. Br J Surg. 1960;47:585–95.
Goligher JC, Hughes ESR. Sensibility of the rectum and colon. Its rôle in the mechanism of anal continence. Lancet. 1951;1(6654):543–7.
Pucciani F, Trafeli M. Sampling reflex: pathogenic role in functional defecation disorder. Tech Coloproctol. 2021;25(5):521–30.
Gallo G, Martellucci J, Sturiale A, et al. Consensus statement of the Italian Society of Colorectal Surgery (SICCR): management and treatment of hemorrhoidal disease. Tech Coloproctol. 2020;24(2):145–64.
Bittorf B, Ringler R, Forster C, et al. Cerebral representation of the anorectum using functional magnetic resonance imaging. Br J Surg. 2006;93(10):1251–7.
Turnbull GK, Hamdy S, Aziz Q, et al. The cortical topography of human anorectal musculature. Gastroenterology. 1999;117(1):32–9.
Sultan AH, Nugent K. Pathophysiology and nonsurgical treatment of anal incontinence. BJOG. 2004;111(Suppl 1):84–90.
Rao SSC, Patel RS. How useful are manometric tests of anorectal function in the management of defecation disorders? Am J Gastroenterol. 1997;92(3):469–75.
Madoff RD, Parker SC, Varma MG, et al. Faecal incontinence in adults. Lancet. 2004;364(9434):621–32.
Green JR, Soohoo SL. Factors associated with rectal injury in spontaneous deliveries. Obstet Gynecol. 1989;73(5 Pt 1):732–8.
Nygaard IE, Rao SS, Dawson JD. Anal incontinence after anal sphincter disruption: a 30-year retrospective cohort study. Obstet Gynecol. 1997;89(6):896–901.
O’Herlihy C. Obstetric perineal injury: risk factors and strategies for prevention. Semin Perinatol. 2003;27(1):13–9.
Dudding TC, Vaizey CJ, Kamm MA. Obstetric anal sphincter injury: incidence, risk factors, and management. Ann Surg. 2008;247(2):224–37.
Oberwalder M, Dinnewitzer A, Baig MK, et al. The association between late-onset fecal incontinence and obstetric anal sphincter defects. Arch Surg. 2004;139:429–32.
Rao SS, Ozturk R, Stessman M. Investigation of the pathophysiology of fecal seepage. Am J Gastroenterol. 2004;99(11):2204–9.
Hellström L, Ekelund P, Milsom I, Skoog I. The influence of dementia on the prevalence of urinary and faecal incontinence in 85-year-old men and women. Arch Gerontol Geriatr. 1994;19(1):11–20.
Davies MC, Creighton SM, Wilcox DT. Long-term outcomes of anorectal malformations. Pediatr Surg Int. 2004;20(8):567–72.
Jóhannsson HO, Påhlman L, Graf W. Randomized clinical trial of the effects on anal function of Milligan-Morgan versus Ferguson haemorrhoidectomy. Br J Surg. 2006;93(10):1208–14.
Bouchoucha M, Devroede G, Faye A, et al. Importance of colonic transit evaluation in the management of fecal incontinence. Int J Colorectal Dis. 2002;17(6):412–7; discussion 418–9
Chan CLH, Lunniss PJ, Wang D, et al. Rectal sensorimotor dysfunction in patients with urge fecal incontinence: evidence from prolonged manometric studies. Gut. 2005;54(9):1263–72.
Santoro GA, Eitan BZ, Pryde A, et al. Open study of low-dose amitriptyline in the treatment of patients with idiopathic fecal incontinence. Dis Colon Rectum. 2000;43(12):1676–81.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits any noncommercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if you modified the licensed material. You do not have permission under this license to share adapted material derived from this chapter or parts of it.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2023 The Author(s)
About this chapter
Cite this chapter
Gallo, G., Realis Luc, A., Trompetto, M. (2023). Epidemiology, Anorectal Anatomy, Physiology and Pathophysiology of Continence. In: Docimo, L., Brusciano, L. (eds) Anal Incontinence. Updates in Surgery. Springer, Cham. https://doi.org/10.1007/978-3-031-08392-1_2
Download citation
DOI: https://doi.org/10.1007/978-3-031-08392-1_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-08391-4
Online ISBN: 978-3-031-08392-1
eBook Packages: MedicineMedicine (R0)