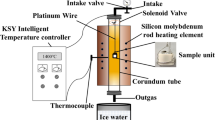
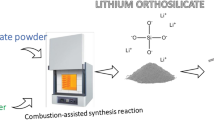
Abstract
Lithium orthosilicate is a candidate material for carbon dioxide adsorption and for the International Thermonuclear Experimental Reactor (ITER) DEMO and future fusion reactor. Solid State Reaction Process (SSRP) is one of the methods for the synthesis of lithium orthosilicate using silicon dioxide and lithium carbonate. In the present study, reaction kinetics of lithium orthosilicate synthesis by SSRP using lithium carbonate and silicon dioxide were studied using non isothermal Thermo-Gravimetric and Differential Thermal Analysis (TG–DTA). TG–DTA data analyzed using different methods for the prediction of kinetic triplet viz. pre-exponential factor (A), activation energy (E) and model for solid state reactions (f(α)). The lithium orthosilicate synthesis reaction of lithium carbonate and silicon-dioxide is controlled by nucleation mechanism for the synthesis of lithium orthosilicate and the best suitable reaction model for this reaction is Avrami–Erofeyev nucleation (A4). The average activation energy and pre-exponential factor calculated using various methods were 568 kJ/mol and 6.45 × 1028 min−1 respectively.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Akahira T, Sunose T (1971) Joint convention of four electrical institutes. Res Rep Chiba Inst Technol (Sci Technol) 16:22–31
Akashi T, Nanko M, Maruyama T, Shiraishi Y, Tanabe J (1998) Solid-state reaction kinetics of LaCrO from the oxides and determination of La 3+ diffusion coefficient. J Electrochem Soc 145(6):2090–2094
Amorim SM, Domenico MD, Dantas TLP, José HJ, Moreira RFPM (2016) Lithium orthosilicate for CO2 capture with high regeneration capacity: kinetic study and modelling of carbonation and decarbonation reactions. Chem Eng J 283:388–396
Augis JA, Bennett JE (1978) Calculation of the Avrami parameters for heterogeneous solid state reactions using a modification of the Kissinger method. J Therm Anal 13:283–293
Boswell PG (1989) On the calculation of activation energies using a modified Kissinger method. J Therm Anal Calorim 18:353–358
Carella E, Hernandez T (2002) Ceramics for fusion reactors: the role of the lithium orthosilicate as breeder. Phys B: Condens Matter 407:4431–4435
Coats AW, Redfern JP (1964) Kinetic parameters from thermogravimetric data. Nature (London) 201:68–90
Flynn JH, Wall LA (1966) A quick direct method for determination of activation energy form thermogravimetric data. J Poly Science B, Poly Lett 4:323–328
Ghuge NS, Mandal D (2013) Kinetics study of solid state reaction for synthesis of lithium titanate by using TG-DTA. CHEMCON-2013
Ghugeand NS, Mandal D (2017) Synthesis of LiDyO2 by solid-state reaction process and study of reaction kinetics by using TG-DTA and XRD techniques. Indian Chem Eng 59:2101–2116
Kenji E, Takehiko M, Masahiro K (2008) Effect of equilibrium-shift in the case of using lithium silicate pellets in ethanol steam reforming. Int J Hydrogen Energy 33:6612–6618
Khawam A, Flanagan DR (2005) Complementary use of model-free and modelistic methods in the analysis of solid-state kinetics. J Phys Chem B 109:10073–17080
Khawam A, Flanagan DR (2006) Solid-state kinetic models: basics and mathematical fundamentals. J Phys Chem B 110:17315–17328
Kissinger HE (1957) Reaction kinetics in differential thermal analysis. Anal Chem 29:1702–1706
Malek J (1992) The kinetic-analysis of nonisothermal data. Thermochim Acta 200:257–269
Mandal D (2014) Reaction kinetics for the synthesis of lithium-titanate (Li2TiO3) by solid state reaction. ARPN J Sci Tech 4(2):59–66
Mandal D, Shenoi MRK, Ghosh SK (2010) Synthesis & fabrication of lithium-titanate pebbles for ITER breeding blanket by solid state reaction & spherodization. Fusion Eng Design 85:819–823
Mandal D, Jadeja MC, Ghuge NS, Sen D, Mazumder S (2016) Effect of excess lithium on sintering behaviour of lithium-titanate pebbles: Modifications of microstructure and pore morphology. Fusion Eng Des 112(15):520–526
Mandal D, Sen D, Mazumder S, Shenoi MRK, Ramnathan S, Sathiyamoorthy D (2011) Sintering behaviour of lithium-titanate pebbles: modifications of microstructure and pore morphology. Wiley, Hoboken, vol 32, no 2, pp 165–170
Mandal D, Sathiyamoorthy D, Rao VG (2012) Preparation and characterization of lithium–titanate pebbles by solid-state reaction extrusion and spherodization techniques for fusion reactor. Fusion Eng Design 87:7–12
Mandal D, Jadeja MC, Chougule BK (2015) Synthesis of lithium orthosilicate and fabrication of pebbles by the solid-state reaction process. IICHE 1–15
Mandal D, Ghuge NS, Jadeja MC (2020) Development and demonstration of a semi-automatic system for the bulk production of lithium titanate (Li2TiO3) pebbles by solid state reaction process (SSRP). Fus Eng Des 159:111871
Ozawa T (1965) A new method of analyzing thermogravimetric data. Bull ChemSocJpn 38:1881–1886
Sonak S, Jain U, Sahu AK, Kumar S, Krishnamurth N (2015) Thermogravimetric analysis and kinetic study of formation of lithium titanate by solid state route. J Nuclear Mater 457:88–93
Vyazovkin S, Burnham AK, Criado JM, Parez-Maqueda LA, Popescu C, Sbirrazzuoli N (2011) ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim Acta 520:1–19
Wang J-X, Chen K-T, Wu, Jhong-Syuan, Wang P-H, Chen C-C (2012) Production of biodiesel through transesterification of soybean oil using lithium orthosilicate solid catalyst. Fuel Process Technol 104:167–173
Yasnó JP, Conconi S, Visintin A (2021) Non-isothermal reaction mechanism and kinetic analysis for the synthesis of monoclinic lithium zirconate (m-Li2ZrO3) during solid-state reaction. J Anal Sci Technol 12:15. https://doi.org/10.1186/s40543-021-00267-5
Acknowledgements
The authors are thankful to, Shri D. R. Avhad, Shri C. A. Shinde, Shri R. Rathore and Shri S. Y. Sarang of Alkali Material & Metal Division, BARC for their constant assistance in this work.
Declaration of Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this paper
Cite this paper
Ghuge, N.S., Mandal, D., Jadeja, M.C., Chougule, B.K. (2022). Kinetics Analysis of Solid State Reaction for the Synthesis of Lithium Orthosilicate. In: Ratan, J.K., Sahu, D., Pandhare, N.N., Bhavanam, A. (eds) Advances in Chemical, Bio and Environmental Engineering. CHEMBIOEN 2021. Environmental Science and Engineering. Springer, Cham. https://doi.org/10.1007/978-3-030-96554-9_25
Download citation
DOI: https://doi.org/10.1007/978-3-030-96554-9_25
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-96553-2
Online ISBN: 978-3-030-96554-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)