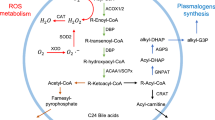
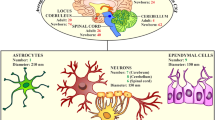
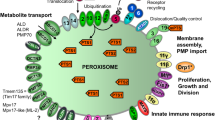
Abstract
Peroxisomes are multifunctional organelles best known for their role in cellular lipid and hydrogen peroxide metabolism. In this chapter, we review and discuss the diverse functions of this organelle in brain physiology and neurodegeneration, with a particular focus on oxidative stress. We first briefly summarize what is known about the various nexuses among peroxisomes, the central nervous system, oxidative stress, and neurodegenerative disease. Next, we provide a comprehensive overview of the complex interplay among peroxisomes, oxidative stress, and neurodegeneration in patients suffering from primary peroxisomal disorders. Particular examples that are discussed include the prototypic Zellweger spectrum disorders and X-linked adrenoleukodystrophy, the most prevalent peroxisomal disorder. Thereafter, we elaborate on secondary peroxisome dysfunction in more common neurodegenerative disorders, including Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis. Finally, we highlight some issues and challenges that need to be addressed to progress towards therapies and prevention strategies preserving, normalizing, or improving peroxisome activity in patients suffering from neurodegenerative conditions.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Notes
- 1.
We apologize to colleagues whose work could not be cited due to space limitations.
Abbreviations
- ABCD:
-
ATP-binding cassette subfamily D
- AD:
-
Alzheimer’s disease
- ALDP:
-
Adrenoleukodystrophy protein
- ALS:
-
Amyotrophic lateral sclerosis
- CNS:
-
Central nervous system
- DAO:
-
D-amino acid oxidase
- DHA:
-
Docosahexaenoic acid
- EAE:
-
Experimental allergic encephalomyelitis
- MS:
-
Multiple sclerosis
- PD:
-
Parkinson’s disease
- PEX:
-
Peroxin
- PPA:
-
Peroxisome-proliferating agent
- PPAR:
-
Peroxisome proliferator-activated receptor
- PUFA:
-
Polyunsaturated fatty acid
- ROS:
-
Reactive oxygen species
- VLCFA:
-
Very-long-chain fatty acid
- X-ALD:
-
X-linked adrenoleukodystrophy
- ZSD:
-
Zellweger spectrum disorder
References
We apologize to colleagues whose work could not be cited due to space limitations.
Islinger M, Voelkl A, Fahimi HD et al (2018) The peroxisome: an update on mysteries 2.0. Histochem Cell Biol 150:443–471
Van Veldhoven PP (2010) Biochemistry and genetics of inherited disorders of peroxisomal fatty acid metabolism. J Lipid Res 51:2863–2895
Lismont C, Revenco I, Fransen M (2019) Peroxisomal hydrogen peroxide metabolism and signaling in health and disease. Int J Mol Sci 20:E3673
Lismont C, Nordgren M, Van Veldhoven PP et al (2015) Redox interplay between mitochondria and peroxisomes. Front Cell Dev Biol 3:35
Di Cara F, Andreoletti P, Trompier D et al (2019) Peroxisomes in immune response and inflammation. Int J Mol Sci 20:E3877
Fransen M, Lismont C, Walton P (2017) The peroxisome-mitochondria connection: how and why? Int J Mol Sci 18:E1126
Schönenberger MJ, Kovacs WJ (2017) Isolation of peroxisomes from mouse brain using a continuous Nycodenz gradient: a comparison to the isolation of liver and kidney peroxisomes. Methods Mol Biol 1595:13–26
Kassmann CM, Quintes S, Rietdorf J et al (2011) A role for myelin-associated peroxisomes in maintaining paranodal loops and axonal integrity. FEBS Lett 585:2205–2211
Berger J, Dorninger F, Forss-Petter S et al (2016) Peroxisomes in brain development and function. Biochim Biophys Acta 1863:934–955
Van Veldhoven PP, Baes M (2013) Peroxisome-deficient invertebrate and vertebrate animal models. Front Physiol 4:335
Cobley JN, Fiorello ML, Bailey DM (2018) 13 reasons why the brain is susceptible to oxidative stress. Redox Biol 15:490–503
Cenini G, Lloret A, Cascella R (2019) Oxidative stress in neurodegenerative diseases: from a mitochondrial point of view. Oxidative Med Cell Longev 2019:2105607
Schlüter A, Real-Chicharro A, Gabaldón T et al (2010) PeroxisomeDB 2.0: an integrative view of the global peroxisomal metabolome. Nucleic Acids Res 38:D800–D805
Wanders RJA (2018) Peroxisomal disorders: improved laboratory diagnosis, new defects and the complicated route to treatment. Mol Cell Probes 40:60–69
Dorninger F, Forss-Petter S, Berger J (2017) From peroxisomal disorders to common neurodegenerative diseases - the role of ether phospholipids in the nervous system. FEBS Lett 591:2761–2788
Brodde A, Teigler A, Brugger B et al (2012) Impaired neurotransmission in ether lipid-deficient nerve terminals. Hum Mol Genet 21:2713–2724
Schönfeld P, Reiser G (2016) Brain lipotoxicity of phytanic acid and very long-chain fatty acids. Harmful cellular/mitochondrial activities in Refsum disease and X-linked adrenoleukodystrophy. Aging Dis 7:136–149
Zarrouk A, Vejux A, Nury T et al (2012) Induction of mitochondrial changes associated with oxidative stress on very long chain fatty acids (C22:0, C24:0, or C26:0)-treated human neuronal cells (SK-NB-E). Oxidative Med Cell Longev 2012:623257
Busanello EN, Lobato VG, Zanatta  et al (2014) Pristanic acid provokes lipid, protein, and DNA oxidative damage and reduces the antioxidant defenses in cerebellum of young rats. Cerebellum 13:751–759
Leipnitz G, Amaral AU, Fernandes CG et al (2011) Pristanic acid promotes oxidative stress in brain cortex of young rats: a possible pathophysiological mechanism for brain damage in peroxisomal disorders. Brain Res 1382:259–265
Borges CG, Canani CR, Fernandes CG et al (2015) Reactive nitrogen species mediate oxidative stress and astrogliosis provoked by in vivo administration of phytanic acid in cerebellum of adolescent rats: a potential contributing pathomechanism of cerebellar injury in peroxisomal disorders. Neuroscience 304:122–132
De Munter S, Verheijden S, Régal L et al (2015) Peroxisomal disorders: a review on cerebellar pathologies. Brain Pathol 25:663–678
ten Brink HJ, van den Heuvel CM, Poll-The BT et al (1993) Peroxisomal disorders: concentrations of metabolites in cerebrospinal fluid compared with plasma. J Inherit Metab Dis 16:587–590
Verhoeven NM, Kulik W, van den Heuvel CM et al (1995) Pre- and postnatal diagnosis of peroxisomal disorders using stable-isotope dilution gas chromatography--mass spectrometry. J Inherit Metab Dis 18(Suppl 1):45–60
Rahim RS, Chen M, Nourse CC et al (2016) Mitochondrial changes and oxidative stress in a mouse model of Zellweger syndrome neuropathogenesis. Neuroscience 334:201–213
Ahlemeyer B, Gottwald M, Baumgart-Vogt E (2012) Deletion of a single allele of the Pex11β gene is sufficient to cause oxidative stress, delayed differentiation and neuronal death in mouse brain. Dis Model Mech 5:125–140
Bottelbergs A, Verheijden S, Van Veldhoven PP et al (2012) Peroxisome deficiency but not the defect in ether lipid synthesis causes activation of the innate immune system and axonal loss in the central nervous system. J Neuroinflammation 9:61
Deon M, Marchetti DP, Donida B et al (2016) Oxidative stress in patients with X-linked adrenoleukodystrophy. Cell Mol Neurobiol 36:497–512
Moser HW, Mahmood A, Raymond GV (2007) X-linked adrenoleukodystrophy. Nat Clin Pract Neurol 3:140–151
Ranea-Robles P, Launay N, Ruiz M et al (2018) Aberrant regulation of the GSK-3β/NRF2 axis unveils a novel therapy for adrenoleukodystrophy. EMBO Mol Med 10:e8604
Singh I, Pujol A (2010) Pathomechanisms underlying X-adrenoleukodystrophy: a three-hit hypothesis. Brain Pathol 20:838–844
Nury T, Zarrouk A, Ragot K et al (2017) 7-Ketocholesterol is increased in the plasma of X-ALD patients and induces peroxisomal modifications in microglial cells: potential roles of 7-ketocholesterol in the pathophysiology of X-ALD. J Steroid Biochem Mol Biol 169:123–136
Jo DS, Cho DH (2019) Peroxisomal dysfunction in neurodegenerative diseases. Arch Pharm Res 42:393–406
Lévy E, El Banna N, Baïlle D et al (2019) Causative links between protein aggregation and oxidative stress: a review. Int J Mol Sci 20:E3896
Sasabe J, Miyoshi Y, Suzuki M et al (2012) D-amino acid oxidase controls motoneuron degeneration through D-serine. Proc Natl Acad Sci U S A 109:627–632
Wojsiat J, Zoltowska KM, Laskowska-Kaszub K et al. (2018) Oxidant/antioxidant imbalance in Alzheimer’s disease: therapeutic and diagnostic prospects. Oxidative Med Cell Longev 2018:6435861
Porcellotti S, Fanelli F, Fracassi A et al (2015) Oxidative stress during the progression of β-amyloid pathology in the neocortex of the Tg2576 mouse model of Alzheimer’s disease. Oxidative Med Cell Longev 2015:967203
Kou J, Kovacs GG, Höftberger R et al (2011) Peroxisomal alterations in Alzheimer’s disease. Acta Neuropathol 122:271–283
Igarashi M, Ma K, Gao F et al (2011) Disturbed choline plasmalogen and phospholipid fatty acid concentrations in Alzheimer’s disease prefrontal cortex. J Alzheimers Dis 24:507–517
Shi Y, Sun X, Sun Y et al (2016) Elevation of cortical C26:0 due to the decline of peroxisomal β-oxidation potentiates amyloid β generation and spatial memory deficits via oxidative stress in diabetic rats. Neuroscience 315:125–135
Inestrosa NC, Carvajal FJ, Zolezzi JM et al (2013) Peroxisome proliferators reduce spatial memory impairment, synaptic failure, and neurodegeneration in brains of a double transgenic mice model of Alzheimer’s disease. J Alzheimers Dis 33:941–959
Nell HJ, Au JL, Giordano CR et al (2016) Targeted antioxidant, catalase-SKL, reduces beta-amyloid toxicity in the rat brain. Brain Pathol 27:86–94
Bose A, Beal MF (2019) Mitochondrial dysfunction and oxidative stress in induced pluripotent stem cell models of Parkinson’s disease. Eur J Neurosci 49:525–532
Marin R, Fabelo N, Martín V et al (2017) Anomalies occurring in lipid profiles and protein distribution in frontal cortex lipid rafts in dementia with Lewy bodies disclose neurochemical traits partially shared by Alzheimer’s and Parkinson’s diseases. Neurobiol Aging 49:52–59
Suzuki K, Iseki E, Togo T et al (2007) Neuronal and glial accumulation of alpha- and beta-synucleins in human lipidoses. Acta Neuropathol 114:481–489
Yakunin E, Moser A, Loeb V et al (2010) Alpha-synuclein abnormalities in mouse models of peroxisome biogenesis disorders. J Neurosci Res 88:866–876
Yakunin E, Loeb V, Kisos H et al (2012) Α-synuclein neuropathology is controlled by nuclear hormone receptors and enhanced by docosahexaenoic acid in a mouse model for Parkinson’s disease. Brain Pathol 22:280–294
Yakunin E, Kisos H, Kulik W et al (2014) The regulation of catalase activity by PPAR γ is affected by α-synuclein. Ann Clin Transl Neurol 1:145–159
Rane P, Sarmah D, Bhute S et al (2019) Novel targets for Parkinson’s disease: addressing different therapeutic paradigms and conundrums. ACS Chem Neurosci 10:44–57
Miville-Godbout E, Bourque M, Morissette M et al (2016) Plasmalogen augmentation reverses striatal dopamine loss in MPTP mice. PLoS One 11:e0151020
Faissner S, Plemel JR, Gold R et al (2019) Progressive multiple sclerosis: from pathophysiology to therapeutic strategies. Nat Rev Drug Discov 18:905–922
Gray E, Rice C, Hares K et al (2014) Reductions in neuronal peroxisomes in multiple sclerosis grey matter. Mult Scler 20:651–659
Singh I, Paintlia AS, Khan M et al (2004) Impaired peroxisomal function in the central nervous system with inflammatory disease of experimental autoimmune encephalomyelitis animals and protection by lovastatin treatment. Brain Res 1022:1–11
Singh I, Samuvel DJ, Choi S et al (2018) Combination therapy of lovastatin and AMP-activated protein kinase activator improves mitochondrial and peroxisomal functions and clinical disease in experimental autoimmune encephalomyelitis model. Immunology 154:434–451
Szalardy L, Zadori D, Bencsik K et al (2017) Unlike PPARgamma, neither other PPARs nor PGC-1alpha is elevated in the cerebrospinal fluid of patients with multiple sclerosis. Neurosci Lett 651:128–133
Gonzalo H, Brieva L, Tatzber F et al (2012) Lipidome analysis in multiple sclerosis reveals protein lipoxidative damage as a potential pathogenic mechanism. J Neurochem 123:622–634
Hossain MS, Abe Y, Ali F et al (2017) Reduction of ether-type glycerophospholipids, plasmalogens, by NF-κB signal leading to microglial activation. J Neurosci 37:4074–4092
Young JM, Nelson JW, Cheng J et al (2015) Peroxisomal biogenesis in ischemic brain. Antioxid Redox Signal 22:109–120
Moruno-Manchon JF, Uzor NE, Kesler SR et al (2018) Peroxisomes contribute to oxidative stress in neurons during doxorubicin-based chemotherapy. Mol Cell Neurosci 86:65–71
Debbabi M, Nury T, Zarrouk A et al (2016) Protective effects of α-tocopherol, γ-tocopherol and oleic acid, three compounds of olive oils, and no effect of Trolox, on 7-ketocholesterol-induced mitochondrial and peroxisomal dysfunction in microglial BV-2 cells. Int J Mol Sci 17:E1973
Acknowledgements
This work was supported by grants from the KU Leuven (C14/18/088), the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen (Onderzoeksprojecten G095315N, G091819N, and GOA8619N), the ERA-Net for Research Programmes on Rare Diseases (PERescue), and H2020-MSCA-ITN (812968). CL is supported by a postdoctoral fellowship of the Research Foundation—Flanders (1213620 N).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Fransen, M., Revenco, I., Li, H., Costa, C.F., Lismont, C., Van Veldhoven, P.P. (2020). Peroxisomal Dysfunction and Oxidative Stress in Neurodegenerative Disease: A Bidirectional Crosstalk. In: Lizard, G. (eds) Peroxisome Biology: Experimental Models, Peroxisomal Disorders and Neurological Diseases. Advances in Experimental Medicine and Biology, vol 1299. Springer, Cham. https://doi.org/10.1007/978-3-030-60204-8_2
Download citation
DOI: https://doi.org/10.1007/978-3-030-60204-8_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-60203-1
Online ISBN: 978-3-030-60204-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)