Summary
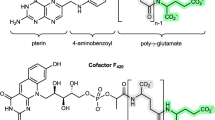
The naturally occurring pteroylpolyglutamate derivatives are substrates for the folate-mediated reactions in cells, including the reactions catalyzed by two multifunctional folate dependent enzymes in eucaryotes. The appropriate derivatives of tetrahydropteroyl (glutamate)n where n = 1, 3, 5, or 7 were used to determine the specificity for, and kinetic advantages of the extra glutamyl residues with two multifunctional proteins from pig liver: methylene-tetrahydrofolate dehydrogenase-methenyltetrahydrofolate cyclohydrolase-formyltetrahydrofolate synthetase, and formiminotransferase-formimotetrahydrofolate cyclodeaminase. Specificity for the polyglutamate derivatives ranged from 10-to 70-fold as indicated from Km values or from the ability to inhibit the five different enzyme activities. With the sequential activities of the transferase-deaminase enzyme, it was demonstrated that when the tetrahydropteroyl pentaglutamate is used as a substrate, the intermediate formimino-compound does not accumulate in the medium. That this kinetic observation is due to preferential transfer of the pentaglutamate- but not monoglutamate intermediate from transferase to deaminase sites without its release from the enzyme molecule was supported by three types of experiments. Chemical modification to yield monofunctional derivatives of the transferase-deaminase affected the kinetics of the recombined activities only with the pentaglutamate substrate, causing a lag in the appearance of final product. Inhibition studies demonstrated that the deaminase activity could preferentially be inhibited only with the monoglutamate substrate. The deaminase activity with the monoglutamate substrate was increased by providing elevated formiminotetrahydrofolate in the assay mixture; no effect was observed when the reaction was carried out with pentaglutamate. Preliminary binding studies indicate a single folate site per subunit of the octameric enzyme, suggesting a type of combined transferase-deaminase site.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Blakely RL (1969) The Biochemistry of Folic Acid and Related Pteridines (Neuberger, A and Tatum EL, eds), American Elsevier, New York.
Baugh CM and Krumdieck CL (1971) Ann. N.Y. Acad. Sci. 186:7–28.
Taylor RT and Hanna ML (1974) Arch. Biochem. Biophys. 181:331–334
Hofftnan RM and Erbe RW (1974) J. Cell Biol. 63:141A.
Scott JM (1976) Biochem. Soc. Trans. 4:845–850.
Reed B, Weir C and Scott JM (1976) Biochem. Soc. Trans. 4:906–907.
Curthoys NP and Rabinowitz JC (1972) J. Biol. Chem. 247:1965–1971.
Kisliuk RL, Gaumont Y and Baugh CM (1974) J. Biol. Chem. 249:4 100–14103.
Coward JK, Chello PH, Cashmore A, Parameswaron KN, De Angelis LM and Bertino JR (1975) Biochemistry 14:1548–1552.
Baggot JE and Krumdieck CL (1979) Biochemistry 18:1036–1041.
Bertino JR, Coward JK, Cashmore A, Chello P, Panichajakul S, Howarth CG and Stout RW (1976) Biochem. Soc. Trans. 4:843–856.
Dolnick BJ and Cheng YC (1978) J. Biol. Chem. 253:3563–3567.
MacKenzie RE and Baugh CM (1980) Biochimica et Biophysica Acta 611:187–195.
Matthews RG and Baugh CM (1980) Biochemistry 19:2040–2045.
Kisliuk RL, Gaumont Y, Lafer E, Baugh CM and Montgomery JA (1981) Biochemistry 20:929–934.
Krumdieck CL, Cornwell PE, Thompson RW and White WE Jr. (1977) Folic Acid, Proc. Workshop, 1975, 25–42.
Caperelli CA, Benkovic PA, Chettur G and Benkovic SJ (1980) J. Biol. Chem. 255:1885–1890.
Smith GK, Mueller WT, Wasserman GF, Taylor WD and Benkovic SJ (1980) Biochemistry 19:4313–4321.
Davis RH (1967) An Organizational Biosynthesis (Vogel HJ, Lampen JO and Bryson V eds.) Academic Press, New York pp. 303–322.
Kirschner K and Bisswanger H (1976) Ann. Rev. Biochem. 45:143–165.
Welch GR (1977) Prog. Biophys. and Mol. Biol. 32:103–191.
Beaudet R and MacKenzie RE (1976) Biochem. Biophys. Acta 453:151–161.
Drury EJ and MacKenzie RE (1977) Can. J. Biochem. 55:919–923.
MacKenzie RE, Aldridge M and Paquin J (1980) J. Biol. Chem. 255:9474–9478.
MacKenzie RE (1973) Biochem. Biophys. Res. Commun. 53:1088–1095.
Paukert JL, D’Ari-Straus L and Rabinowitz JC (1976) J. Biol. Chem. 251:5104–5111.
Tan LUL, Drury EJ and MacKenzie RE (1977) J. Biol. Chem. 252:1117–1122.
Cohen L and MacKenzie RE (1978) Biochem. Biophys. Acta 522:311–317.
Krumdieck CL and Baugh CM (1969) Biochemistry 8:1568–1572.
Drury EJ, Bazar LS and MacKenzie RE (1975) Arch. Biochem. Biophys. 169:662–668.
MacKenzie RE (1980) Methods in Enzymology 66:626–630.
MacKenzie RE and Tan LUL (1980) Methods in Enzymology 66:609–626.
Blakely RL (1957) Biochan. J. 65:331–322.
Blakely, RL (1960) Nature 188:231–232.
Paulus H (1969) Biochemistry 32:91 – 100.
Cantley LC and Hammes GG (1973) Biochemistry 12:4900–4904.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1983 Springer Science+Business Media New York
About this chapter
Cite this chapter
MacKenzie, R.E., Baugh, C.M. (1983). Interaction of Tetrahydropteroylpolyglutamates with Two Folate-Dependent Multifunctional Enzymes. In: Goldman, I.D., Chabner, B.A., Bertino, J.R. (eds) Folyl and Antifolyl Polyglutamates. Advances in Experimental Medicine and Biology, vol 163. Springer, Boston, MA. https://doi.org/10.1007/978-1-4757-5241-0_2
Download citation
DOI: https://doi.org/10.1007/978-1-4757-5241-0_2
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4757-5243-4
Online ISBN: 978-1-4757-5241-0
eBook Packages: Springer Book Archive