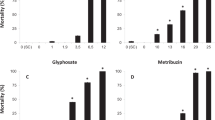
Abstract
Anurans from the genus Xenopus have long been used as standard testing organisms and occur naturally in tropical and sub-tropical areas where malaria vector control pesticides are actively used. However, literature on the toxic effects of these pesticides is limited. This review analyses the available data pertaining to both Xenopus and the pesticides used for malaria vector control in order to determine the pesticides that have the greatest potential to influence amphibian health while also identifying gaps in literature that need to be addressed. Amphibian diversity has shown the fastest decline of any group, yet there are still voids in our understanding of how this is happening. The lack of basic toxicity data on amphibians with regard to pesticides is an issue that needs to be addressed in order to improve effectiveness of amphibian conservation strategies. Meta-analyses performed in this review show that, at current usage, with the available acute toxicity literature, the pyrethroid pesticide group could hold the highest potential to cause acute toxicity to Xenopus sp. in relation to the other MVCPs discussed, but the lack of data cripples the efficacy with which meta-analyses can be performed and conclusions made from such analyses. Several studies have shown that DDT accumulates in Xenopus sp. from malaria vector control areas, but accumulation of other MVCPs in frogs is still largely unknown. Through this review we hope to encourage future research into the field of amphibian ecotoxicology and to promote the use of the Xenopus standard model in order to build comprehensive datasets that may be used in amphibian conservation.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- ACP:
-
Acid phosphatase
- AI:
-
Active ingredient
- AMA:
-
Amphibian metamorphosis assay
- CaE:
-
Carboxylesterase
- CSA:
-
Cockayne syndrome A gene
- DDA:
-
Dichlorodiphenylacetic acid
- DDD:
-
Dichlorodiphenyldichloroethane
- DDE:
-
Dichlorodiphenyldichloroethylene
- DDT:
-
Dichlorodiphenyltrichloroethane
- DNA:
-
Deoxyribonucleic acid
- EC50:
-
Effective concentration where 50% of the test population are affected
- EDC:
-
Endocrine-disrupting compound
- FETAX:
-
Frog embryo teratogenesis assay-Xenopus
- GST:
-
Glutathione-S-transferase
- IC50:
-
Inhibition concentration where 50% of the test population show inhibition of a measured aspect
- IRS:
-
Indoor residual spraying
- ITN:
-
Insecticide-treated net
- LAGDA:
-
Larval amphibian growth and development assay
- LC50:
-
Lethal concentration where 50% of the test population died
- LDH:
-
Lactate dehydrogenase
- LOEC:
-
Lowest observed effects concentration
- MCIG:
-
Minimum concentration to inhibit growth
- m-RNA:
-
Messenger ribonucleic acid
- MUTL:
-
Muir-Torre syndrome gene
- MVC:
-
Malaria vector control
- MVCP:
-
Malaria vector control pesticide
- NAD+ :
-
Nicotinamide adenine dinucleotide
- NF:
-
Nieuwkoop-Faber
- OECD:
-
Organisation for Economic Co-operation and Development
- POP:
-
Persistent organic pollutant
- SSD:
-
Species sensitivity distribution
- TI:
-
Teratogenic index
- UVB:
-
Ultraviolet B
- WHO:
-
World Health Organization
- XPA:
-
Xeroderma pigmentosum group A gene
- XPG:
-
Xeroderma pigmentosum group G gene
References
Akkermans LMA, Van den Bercken J, Versluijs-Helder M (1975) Comparative effects of DDT, allethrin, and aldrin-transdiol on sense organs of Xenopus laevis. Pest Biochem Physiol 5:451–457
Alonso P, Noor AM (2017) The global fight against malaria is at crossroads. Lancet 390:2532–2534
Ansara-Ross TM, Wepener V, Van den Brink PJ, Ross MJ (2012) Pesticides in South African fresh waters. Afr J Aquat Sci 37:1–16
Århem P, Frankenhaeuser B (1974) DDT and related substances: effects on permeability properties of myelinated Xenopus nerve fibre. Potential clamp analysis. Acta Physiol Scand 91:502–511
Århem P, Frankenhaeuser B, Göthe R, O’Bryan P (1974) DDT and related substances on myelinated nerve: effects on permeability properties. Acta Physiol Scand 91:130–132
ASTM (American Society for Testing and Materials) (2012) Standard guide for conducting the frog embryo teratogenesis assay – Xenopus (FETAX). Annual book of ASTM standards. American Society for Testing and Materials, Philadelphia
ATSDR (Agency for Toxic, Substances and Disease Registry) (2002) Toxicological profile for DDT, DDE, and DDD. Agency for Toxic, Substances and Disease Registry. U.S. Department of Health and Human Services, Atlanta. http://www.atsdr.cdc.gov/toxprofiles/tp35.pdf. Accessed Aug 2018
Avila VL, Frye PG (1978) Feeding behaviour of the African clawed frog (Xenopus Laevis Daudin): (Amphibia, Anura, Pipidae): effect of prey type. J Herpetol 12:391–396
Aydin-Sinan H, Güngördü A, Ozmen M (2012) Toxic effects of deltamethrin and λ-cyhalothrin on Xenopus laevis tadpoles. J Environ Sci Health B 47:397–402
Bollmohr S, Day JA, Schulz R (2007) Temporal variability in particle associated pesticide exposure in a temporarily open estuary, Western Cape, South Africa. Chemosphere 68:479–488
Bonfanti P, Colombo A, Orsi F, Nizzetto I, Andrioletti M, Bachetta R, Mantecca P, Fascio U, Vailati G, Vismara C (2004) Comparative teratogenicity of chlorpyifos and malathion on Xenopus laevis development. Aquat Toxicol 70:189–200
Bouwman H, Van den Berg H, Kylin H (2011) DDT and malaria prevention: addressing the paradox. Environ Health Perspect 119:744–747
Brod S, Brooks L, Garner TWJ (2018) Discussing the future of amphibians in research. Lab Anim. https://doi.org/10.1038/s41684-018-0193-6
Burggren WW, Warburton S (2007) Amphibians as animal models for laboratory research in physiology. ILAR J 48:260–269
Channing A (1998) Tadpoles as bio-indicators of stream quality: a baseline study. Water Research Commission of South Africa (WRC) Technical Report No. 718/1/98
Chemotti DC, Davis SN, Cook LW, Willoughby IR, Paradise CJ, Lom B (2006) The pesticide malathion disrupts Xenopus and Zebrafish embryogenesis: an investigative laboratory exercise in developmental toxicology. Biocene 32:3–18
Chen Y, Yua K, Hassan M, Xu C, Zhang B, Gin KY, He Y (2018) Occurrence, distribution and risk assessment of pesticides in a river reservoir system. Ecotox Environ Safe 166:320–327
Dabrowski JM, Peall SKC, Reinecke AJ, Liess M, Schulz R (2002) Runoff-related pesticide input into the Lourens River, South Africa: basic data for exposure assessment and risk mitigation at the catchment scale. Water Air Soil Pollut 135:265–283
Daka PS, Obuseng VC, Torto N, Huntsman-Mapila P (2006) Deltamethrin in sediment samples of the Okavango Delta, Botswana. Water SA 32:483–488
Dalu T, Weyl OLF, Froneman PW, Wasserman RJ (2016) Trophic interactions in an austral temperate ephemeral pond inferred using stable isotope analysis. Hydrobiologia 768:81–94
Dumont JN, Schultz TW, Buchanan MV, Kao GL (1983) Frog embryo teratogenesis assay: Xenopus (FETAX) – a short-term assay applicable to complex environmental mixtures. In: Waters MD, Sandhu SS, Lewtas J, Claxton L, Chernoff N, Nesnow S (eds) Short-term bioassays in the analysis of complex environmental mixtures III. Environmental science research, vol 27. Springer, Boston, pp 393–405
Elliot-Feeley E, Armstrong JB (1982) Effects of fenitrothion and carbaryl on Xenopus laevis development. Toxicology 22:319–335
FEOW (Freshwater Ecoregions of the World) (2008) Freshwater amphibian species richness map. http://www.feow.org/maps/biodiversity/freshwater_amphibian_species_richness. Accessed Sept 2018
Glunt KD, Blanford JI, Paaijmans KP (2013) Chemicals, climate, and control: increasing the effectiveness of malaria vector control tools by considering relevant temperatures. PLoS Pathog 9:e1003602
Gonzalez-Mille DJ, Espinosa-Eyes G, Rivero-Péres NE, Trejo-Acevedo A, Nava-montes AD, Ilizaliturri-Hernández CA (2013) Persistent organochlorine pollutants (POPs) and DNA damage in Giant Toads (Rhinella marina) from an industrial area at Coatzacoalcos. Mexico Water Air Soil Pollut 224:1781
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Gurdon JB, Hopwood N (2000) The introduction of Xenopus laevis into developmental biology: of empire, pregnancy testing and ribosomal genes. Int J Dev Biol 44:43–50
Harril JA, Meacham CA, Shafer TJ, Hughes MF, Crofton KM (2005) Time- and concentration-dependent accumulation of [3H]-deltamethrin in Xenopus laevis oocytes. Toxicol Lett 157:79–88
Harris M, Bishop CA, Struger J, Van Den Heuvel MR, Van Der Kraak GJ, Dixon DG, Ripley B, Bogart JP (1998) The functional integrity of northern leopard frog (Rana pipiens) and green frog (Rana clamitans) populations in orchard wetlands I. Genetics physiology, and biochemistry of breeding adults and young-of-the-year. Environ Toxicol Chem 17:1338–1350
Henry L, Kishimba MA (2003) Levels of pesticide residues in water, soil and sediments from southern Lake Victoria and its basin. Tanz J Sci 29:77–89
Hoffmann F, Kloas W (2016) p,p′-Dichlorodiphenyldichloroethylene (p,p′-DDE) can elicit antiandrogenic and estrogenic modes of action in the amphibian Xenopus laevis. Physiol Behav 167:172–178
Hothem RL, Crayon JJ, Law MA (2006) Effects of contaminants on reproductive success of aquatic birds nesting at Edwards Air Force Base, California. Ach Environ Contam Toxicol 51:711–719
Hu L, Zhu J, Rotchell JM, Wu L, Gao J, Shi H (2015) Use of the enhanced frog embryo teratogenesis assay-Xenopus (FETAX) to determine chemically-induced phenotypic effects. Sci Total Environ 508:258–265
Ji Q, Lee J, Lin Y, **g G, Tsai LJ, Chen A, Hetrick L, Jocoy D, Liu J (2016) Atrazine and malathion shorten the maturation process of Xenopus laevis oocytes and have an adverse effect on early embryo development. Toxicol In Vitro 32:63–69
Knaak JB, Dary CC, Zhang X, Gerlach RW, Tornero-Velez R, Chang DT, Goldsmith R, Blancato JN (2012) Parameters for pyrethroid insecticide QSAR and PBPK/PD models for human risk assessment. Rev Environ Contam Toxicol 219:1–114
Lambert MRK (1997) Effects of pesticides on amphibians and reptiles in sub-Saharan Africa. Rev Environ Contam Toxicol 150:31–73
Lambert MRK (2001) Death from pesticides reviewed among non-target amphibians in sub-Saharan Africa. Herpetol Bull 78:21–27
Lillicrap A, Belanger S, Burden N, Du Pasquier D, Embry MR, Halder M, Lampi MA, Lee L, Norberg-King T, Rattner BA (2016) Alternative approaches to vertebrate ecotoxicity tests in the 21st century: a review of developments over the last two decades and current status. Environ Toxicol Chem 35:2637–2646
Lindholm M, Hesen DO, Mosepele K, Wolski P (2007) Food web and energy fluxes on a seasonal floodplain: the influence of flood size. Wetlands 27:775–784
Lutz I, Kloas W (1999) Amphibians as a model to study endocrine disruptors: I. Environmental pollution and estrogen receptor binding. Sci Total Environ 225:49–57
Mann RM, Hyne RV, Choung CB, Wilson SP (2009) Amphibians and agricultural chemicals: review of the risks in a complex environment. Environ Pollut 157:2903–2927
MAP (Malaria Atlas Project) (2018) Temperature suitability index for Plasmodium vivax/falciparum transmission. https://map.ox.ac.uk/explorer/#/explorer. Accessed Sept 2018
Martini F, Fernández C, Segundo LS, Tarzona JV, Pablos VM (2010) Assessment of potential immunotoxic effects caused by cypermethrin, fluoxetine, and thiabendazole using heat shock protein 70 and interleukin-1β mRNA expression in the anuran Xenopus laevis. Environ Toxicol Chem 29:2536–2543
Nieuwkoop PD, Faber J (1994) Normal table of Xenopus laevis (daudin): a systematical & chronological survey of the development from the fertilized egg till the end of metamorphosis. Garland Science, Oxford
Ogbeide O, Chukwuka A, Tongo I, Ezemonye L (2018) Relationship between geosorbent properties and field-based partition coefficients for pesticides in surface water and sediments of selected agrarian catchments: implications for risk assessment. J Environ Manag 217:23–17
Olutona GO, Olatunji SO, Obisanya JF (2016) Downstream assessment of chlorinated organic compounds in the bed-sediment of Aiba Stream, Iwo, South-Western, Nigeria. Springerplus 5:67. https://doi.org/10.1186/s40064-016-1664-0
Palmer BD, Palmer SK (1995) Vitellogenin induction by xenobiotic estrogens in the red-eared turtle and African clawed frog. Environ Health Perspect 103:19–25
Pan D, Liang X (1993) Safety study of pesticide on Bog Frog, a predatory natural enemy of pest in paddy field. J Hunan Agric College 19:47–54
Pauli BD, Perrault JA, Money SL (2000) RATL: a database of reptile and amphibian toxicology literature. Technical Report Series No. 357. Canadian Wildlife Service. Québec, Canada
Robert J, Ohta Y (2009) Comparative and developmental study of the immune system in Xenopus. Dev Dyn 238:1249–1270
Rudek Z, Rożek M (1992) Induction of micronuclei in tadpoles of Rana temporaria and Xenopus laevis by the pyrethroid Fastac 10 EC. Mutat Res 298:25–29
Ruigt GSF, Van den Bercken J (1986) Action of pyrethroids on a nerve-muscle preparation of the Clawed Frog, Xenopus laevis. Pest Biochem Physiol 25:176–187
Saka M (2004) Developmental toxicity of p,p′-dichlorodiphenyltrichloroethane, 2,4,6-trinitrotoluene, their metabolites, and benzo[a]pyrene in Xenopus laevis embryos. Environ Toxicol Chem 23:1065–1073
Sereda BL, Meinhardt HR (2003) Insecticide contamination of the water environment in malaria endemic areas of KwaZulu-Natal (SA): the risk of insecticide resistance development in malaria vectors. WRC Report No. 1119/1/03. Pretoria: Water Research Commission
Sibali LL, Okwonkwo JO, McCrindle RI (2008) Determination of selected organochlorine pesticide (OCP) compounds from the Jukskei River catchment area in Gauteng. South Africa Water SA 34:611–622
Snawder JE, Chambers JE (1989) Toxic and developmental effects of organophosphorus insecticides in embryos of the South African Clawed Frog. J Environ Sci Health B 24:205–218
Snawder JE, Chambers JE (1990) Critical time periods and the effect of tryptophan in malathion-induced developmental defects in Xenopus laevis. Life Sci 46:1635–1642
Snawder JE, Chambers JE (1993) Osteolathyrogenic effects of malathion in Xenopus embryos. Toxicol Appl Pharmacol 121:210–216
Tang W, Wang D, Wang J, Wu Z, Li L, Huang M, Xu S, Yan D (2018) Pyrethroid pesticide residues in the global environment: an overview. Chemosphere 191:990–1007
Thiere G, Schulz R (2004) Runoff-related agricultural impact in relation to macroinvertebrate communities of the Lourens River, South Africa. Water Res 38:3092–3102
UNEP (2009) Stockholm Convention on Persistent Organic Pollutants (POPs), text and annexes as amended in 2009. https://www.wipo.int/edocs/lexdocs/treaties/en/unep-pop/trt_unep_pop_2.pdf. Accessed Feb 2019
Van den Bercken J, Akkermans LMA, Van der Zalm JM (1973a) DDT-like action of allethrin in the sensory nervous system of Xenopus laevis. Eur J Pharmacol 21:95–106
Van den Bercken J, Akkermans LMA, Van Langen RG (1973b) The effect of DDT and dieldrin on skeletal muscle fibres. Eur J Pharmacol 21:89–94
Van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Chem 13:57–149
Van Dyk JC, Bouwman H, Barnhoorn IEJ, Bornman MS (2010) DDT contamination from indoor residual spraying for malaria control. Sci Total Environ 408:2745–2752
Vijverberg HPM, Van den Bercken J (1979) Frequency-dependent effects of the pyrethroid insecticide decamethrin in frog myelinated nerve fibres. Eur J Pharmacol 58:501–504
Vijverberg HPM, Van den Bercken J (1982) Action of pyrethroid insecticides on the vertebrate nervous system. Neuropathol Appl Neurobiol 8:421–440
Vijverberg HPM, Ruigt GSF, Van den Bercken J (1982a) Structure-related effects of pyrethroid insecticides on the lateral-line sense organ and on peripheral nerves of the Clawed Frog, Xenopus laevis. Pest Biochem Physiol 18:315–324
Vijverberg HPM, Van der Zalm JM, Van den Bercken J (1982b) Similar mode of action of pyrethroids and DDT on sodium channel gating in myelinated nerves. Nature 295:601–603
Vijverberg HPM, Van der Zalm JM, Van Kleef RGDM, Van den Bercken J (1983) Temperature- and structure-dependent interaction of pyrethroids with the sodium channels in frog node of Ranvier. Biochim Biophys Acta 728:73–82
Viljoen IM, Bornman R, Bouwman H (2016) DDT exposure to frogs: a case study from Limpopo Province, South Africa. Chemosphere 159:335–341
Walshe DP, Garner P, Adeel AA, Pyke GH, Burkot TR (2017) Larvivorous fish for preventing malaria transmission. Cochrane Database Syst Rev 12:CD008090
Watkins JA, Mecham CA, Crofton KM, Shafer TJ (2007) Concentration-dependent accumulation of [3H]-deltamethrin in sodium channel Nav1.2/β1 expressing Xenopus laevis oocytes. Toxicol In Vitro 21:1672–1677
Webb C, Crain DA (2006) Effects of ecologically relevant doses of malathion on develo** Xenopus laevis tadpoles. Bios 77:1–6
WHO (World Health Organization) (1971) Alternative insecticides for vector control. World Health Org Bull 44:1–446
WHO (World Health Organization) (2006) Indoor residual spraying: use of indoor spraying for scaling up global malaria control and elimination. http://whqlibdoc.who.int/hq/2006/WHO_HTM_MAL_2006.1112_eng.pdf. Accessed Aug 2018
Wolmarans NJ, Wepener V, Du Preez LH, Ikenaka Y, Ishizuka M, Smit NJ (2015) Assessment of the food web structure of Xenopus muelleri from the Phongolo River floodplain using stable isotope analysis. Peer reviewed conference proceedings of the 7th international toxicology symposium in Africa; 31 August 2015, Johannesburg, South Africa
Wolmarans NJ, Du Preez LH, Yohannes YB, Ikenaka Y, Ishizuka M, Smit NJ, Wepener V (2018) Linking organochlorine exposure to biomarker response patterns in anurans: a case study of Müller’s clawed frog (Xenopus muelleri) from a tropical malaria vector control region. Ecotoxicology 27:1203–1216
Yamauchi K, Eguchi R, Shimada N, Ishihara A (2002) The effects of endocrine-disrupting chemicals on thyroid hormone binding to Xenopus laevis transthyretin and thyroid hormone receptor. Clin Chem Lab Med 40:1250–1256
Yu S, Wages M, Cai Q, Maul JD, Cobb GP (2013) Lethal and sublethal effects of three insecticides on two developmental stages of Xenopus laevis and comparison with other amphibians. Environ Toxicol Chem 32:2056–2064
Yu S, Weir SM, Cobb GP, Maul JD (2014) The effects of pesticide exposure on ultraviolet-B radiation avoidance behavior in tadpoles. Sci Total Environ 481:75–80
Yu S, Tang S, Mayer GD, Cobb GP, Maul JD (2015a) Interactive effects of ultraviolet-B radiation and pesticide exposure on DNA photo-adduct accumulation and expression of DNA damage and repair genes in Xenopus laevis embryos. Aquat Toxicol 159:256–266
Yu S, Wages M, Willming M, Cobb GP, Maul JD (2015b) Joint effects of pesticides and ultraviolet-B radiation on amphibian larvae. Environ Pollut 207:248–255
Zippel KC, Mendelson JR (2008) The amphibian extinction crisis: a call to action. Herpetol Rev 39:23–29
Acknowledgements
This study was partially funded by the Flemish Interuniversity Council (VLIR) to ECN (VLIR-OUS project – ZEIN21013PR396), and financial assistance was also provided by the South African National Research Foundation (NRF: Grant no. SFH150624120779). Opinions expressed and conclusions arrived at are those of the authors and are not necessarily to be attributed to VLIR or the NRF.
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Wolmarans, N.J., Bervoets, L., Meire, P., Wepener, V. (2019). Current Status and Future Prognosis of Malaria Vector Control Pesticide Ecotoxicology and Xenopus sp.. In: de Voogt, P. (eds) Reviews of Environmental Contamination and Toxicology Volume 252. Reviews of Environmental Contamination and Toxicology, vol 252. Springer, Cham. https://doi.org/10.1007/398_2019_35
Download citation
DOI: https://doi.org/10.1007/398_2019_35
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-30991-6
Online ISBN: 978-3-030-30992-3
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)