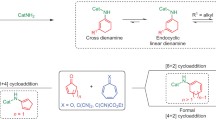
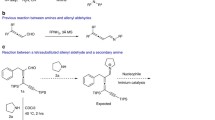
Abstract
Strain in small rings has evolved as one of the principles used to control reactivity and chemoselectivity in transformations of organic compounds. The combination of small rings with multiple bonds and functional groups establishes composite functionalities which demonstrate unique multiple reactivity and thereby potentially high synthetic utility. This survey concentrates on a family of compounds which combines the chemistry of methylenecyclopropanes and that of electron-acceptor-activated alkenes, namely alkyl 2-chloro-2-cyclopropylideneacetates of types 1–3. This special feature makes the compounds 1–3 multifunctional as well as highly reactive, and thus extremely versatile building blocks for organic synthesis. Not only is the general synthetic access to methylenecyclopropanes 1–3 presented here, but particularly their rich chemistry as highly reactive Michael acceptors, dienophiles, dipolarophiles and general cyclophiles which leads to a wide range of different types of functionally substituted cyclopropane derivatives, spirocyclopropane-annelated hetero- and carbocycles, mono- and oligocondensed cycles, natural and unnatural amino acids and peptidomimetics, and more. Finally, the first results obtained with polymer-bound substrates of types 1–3 in a combinatorial approach to libraries of potentially biologically active compounds are presented.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
de Meijere A (ed) (1997) Houben-Weyl, Thieme, Stuttgart, vol E 17a–c
a) de Meijere A (ed) (1986) Small ring compounds in organic synthesis I. Top Curr Chem 133. Springer, Berlin Heidelberg New York; b) de Meijere A (ed) (1987) Small ring compounds in organic synthesis II. Top Curr Chem 135. Springer, Berlin Heidelberg New York; c) Rappoport Z (ed) (1987) The chemistry of the cyclopropyl group. Wiley, Chichester; d) de Meijere A (ed) (1988) Small ring compounds in organic synthesis III. Top Curr Chem 144. Springer, Berlin Heidelberg New York; e) de Meijere A, Blechert S (eds) (1989) Strain and its implications in organic chemistry. NATO-ASI Series C, Kluwer, Dordrecht, vol 273; f) de Meijere A (ed) (1990) Small ring compounds in organic synthesis IV. Top Curr Chem 155. Springer, Berlin Heidelberg New York; g) Wong HNC, Hon M-Y, Tse C-W, Yip Y-C, Tanko J, Hudlicky T (1989) Chem Rev 89:165; h) de Meijere A (ed) (1996) Small ring compounds in organic synthesis V. Top Curr Chem 178. Springer, Berlin Heidelberg New York
Trost BM (1989) In: de Meijere A, Blechert S (eds) Strain and its implications in organic chemistry. NATO-ASI Series C, Kluwer, Dordrecht, vol 273, p 1ff
a) Binger P, Büch HM, Top Curr Chem (1987) 135:77; b) Binger P, Schmidt T (1997) In: de Meijere A (ed) Houben-Weyl, Thieme, Stuttgart, vol E 17c, p 2217; c) Brandi A, Goti A (1998) Chem Rev 98:589; d) de Meijere A, Kozhushkov SI, Khlebnikov AF (1999) Top Curr Chem, this volume
For previous reviews of our own work in this area see: a) de Meijere A (1987) Chemistry in Britain 23:865; b) de Meijere A, Wessjohann L (1990) Synlett 20; c) Mißlitz U, de Meijere A (1989) In: Regitz M, Hanack M (eds) Houben-Weyl, Thieme, Stuttgart, vol 4, p 664 and 769; d) de Meijere A (1992) In: Yoshida Z, Ohshiro Y (eds) New Aspects of Organic Chemistry II. Kodansha-VCH, Tokyo-Weinheim, p 181
a) Stohlmeier M (1988) Dissertation, Universität Hamburg; b) Banwell MG, Corbett M, Gulbis J, MacKay MF, Reum ME (1993) J Chem Soc Perkin Trans 1:945
a) Weber A, de Meijere A (1980) Angew Chem 92:135 Angew Chem Int Ed Engl 19:138; b) Liese T, Splettstößer G, de Meijere A (1982) Tetrahedron Lett 23:3341; c) Liese T, de Meijere A (1982) Angew Chem 94:65 Angew Chem Int Ed Engl 21:65; d) Weber W, de Meijere A (1985) Chem Ber 118:2450; e) Liese T, de Meijere A (1986) Chem Ber 119:2995; f) Liese T, Jaekel F, de Meijere A (1990) Org Synth 69:144; g) Liese T (1983) Dissertation, Universität Hamburg; h) Seyed-Mahdavi F (1986), Dissertation, Universität Hamburg; i) Wenck H (1986) Dissertation, Universität Hamburg; j) de Meijere A, Kozhushkov SI (unpublished results); k) Kordes M (1999) Dissertation, Universität Göttingen; l) Michaelsen H (1994) Dissertation, Universität Göttingen; m) Wessjohann L (1990) Dissertation, Universität Hamburg
Wessjohann L, Giller K, Zuck B, Skattebøl L, de Meijere A (1993) J Org Chem 58:6442
Wessjohann L, Krass N, Yu D, de Meijere A (1992) Chem Ber 125:867
a) Es-Sayed M, Gratkowski C, Krass N, Meyers AI, de Meijere A (1993) Tetrahedron Lett 34:289; b) Gratkowski C (1994) Dissertation, Universität Göttingen; c) Es-Sayed M (1992) Dissertation, Universität Hamburg
a) Ricker CB (1996) Diplomarbeit, Universität Göttingen; b) Ricker CB (1999) Dissertation, Universität Göttingen; c) Labahn T, Noltemeyer M, Heiner T, Ricker CB, de Meijere A (1999) Acta Cryst C (to be submitted)
a) Glück C, Poignée V, Schwager H (1987) Synthesis: 260; b) Sepiol J, Soulen RL (1975) J Org Chem 40:3791
Kostikov RR, de Meijere A (1984) J Chem Soc Chem Commun 1528
In the few known cases of intermolecular reactions of thermally or photochemically generated vinylcarbenes, the yields are markedly lower: a) Franck-Neumann M, Lohmann JJ (1977) Angew Chem 89:331 Angew. Chem Int Ed Engl 16:323 Angew Chem Suppl 1715; b) Stang PJ (1978) Chem Rev 78:383; c) Franck-Neumann M, Lohmann JJ (1979) Tetrahedron Lett 2397; d) Bruce MI (1991) Chem Rev 91:197
a) Liese T, Splettstößer G, de Meijere A (1982) Angew Chem 94:799 Angew Chem Int Ed Engl 21:790; b) Liese T, Teichmann S, de Meijere A (1988) Synthesis 25
Liese T, Seyed-Mahdavi F, de Meijere A (1990) Org Synth 69:148
Our recent experience allows one to increase the yield of this transformation up to 85% if this first SN2′-type replacement has been performed at 20°C for 24 h followed by heating under reflux. Ricker CR, Rauch K (unpublished results)
a) Salaün J, Bennani F, Compain J-C, Fadel A, Ollivier J (1980) J Org Chem 45:4129; b) Spitzner D, Swoboda H (1986) Tetrahedron Lett 27:1281; c) Osborne NF (1982) J Chem Soc Perkin Trans 1:1435
a) de Meijere A, Teichmann S, Seyed-Mahdavi F, Kohlstruk S (1996) Liebigs Ann 1989; b) Kohlstruk S (1992) Dissertation, Universität Göttingen
Seebach D, Hungerbühler E, Naef R, Schnurrenberger P, Weidmann B, Züger M (1982) Synthesis: 138; b) Krass N, Wessjohann L, Yu D, de Meijere A (1989) In: de Meijere A, Blechert S (eds) Strain and its Implications in Organic Chemistry. NATO-ASI Series C, Kluwer Academic Publ, Dordrecht, p 509
a) de Meijere A, Ernst K, Zuck B, Brandl M, Kozhushkov SI, Tamm M, Yufit DS, Howard JAK, Labahn T (1999) Eur J Org Chem in press; b) Ernst K (1994) Dissertation, Universität Göttingen
a) Brandl M, de Meijere A (unpublished results); b) Sokolov VI, de Meijere A (unpublished results); c) Rauch K, de Meijere A (unpublished results)
de Meijere A (1984) Bull Soc Chim Belg 93:241
Bengtson G (1985) Dissertation, Universität Hamburg
a) Michalski SA (1989) Diplomarbeit, Universität Hamburg; b) Michalski SA (1992) Dissertation, Universität Hamburg
a) de Meijere A, Teichmann S, Yu D, Kopf J, Oly M, von Thienen N (1989) Tetrahedron 45:2957; b) Teichmann S (1988) Dissertation, Universität Hamburg
Seyed-Mahdavi F, Teichmann S, de Meijere A (1986) Tetrahedron Lett 27:6185
Spitzner D, Engler A, Wagner P, de Meijere A, Bengtson G, Simon A, Peters K, Peters E-M (1987) Tetrahedron 43:3213
de Meijere A, Wenck H, Seyed-Mahdavi F, Viehe HG, Gallez V, Erden I (1986) Tetrahedron 42:1291
Heiner T (1996) Dissertation, Universität Göttingen
Dolbier WR Jr, Lomas D, Garza T, Harmon C, Tarrant P (1972) Tetrahedron 28:3185
Stella L, Janousek Z, Merényi R, Viehe HG (1978) Angew Chem 90:741 Angew Chem Int Ed Engl 17:691
Yu D (1989) Dissertation, Universität Hamburg
Narasaka K, Soai K, Mukaiyama T (1974) Chem Lett 1223
Conditions as optimized by Paulsen et al: Paulsen H, Hölck J-P (1982) Liebigs Ann Chem 1121. Cf. also [74]
a) Staudinger H, Meyer J (1919) Helv Chim Acta 2:635; b) Review: Gololobov YuG, Kasukhin LF (1992) Tetrahedron 48:1353; c) Mitchell D, Liebeskind LS (1990) J Am Chem Soc 112:291
Reviews: a) Subramanian LR, Zeller K-P (1997) In: de Meijere A (ed) Houben-Weyl, vol E 17a, Thieme, Stuttgart, p 256; b) Simmons HE, Cairns TL, Vladuchik SA, Hoiness CM (1973) Org React 20:1
a) Corey EJ, Chaykovsky M (1962) J Am Chem Soc 84:3782; b) Corey EJ, Chaykovsky M (1965) J Am Chem Soc 87:1353; c) Asunskis J, Shechter H (1968) J Org Chem 33:1164; d) Landor SR, Punja N (1967) J Chem Soc C 2495
de Meijere A, Kozhushkov SI, Yufit DS, Boese R, Haumann T, Pole DL, Sharma PK, Warkentin J (1996) Liebigs Ann 601
Regitz M, Heydt H (1984) In: Padwa A (ed) 1, 3-Dipolar cycloaddition chemistry, Wiley, New York, vol 1, p 393
Aspartt-Pascot L, Bastide J (1971) Comptes Rendus Acad Sci Ser C 273:1772
Moffat JB (1978) J Phys Chem 82:1083
a) Zorn C, Goti A, Brandi A, Johnsen K, Kozhushkov SI, de Meijere A (1998) Chem Commun: 903; b) Zorn C, Goti A, Brandi A, Johnsen K, Noltemeyer M, Kozhushkov SI, de Meijere A (1999) J Org Chem 64:755
a) Danishefsky S (1979) Acc Chem Res 12:66; b) Loreto MA, Pellacani L, Tardella PA (1979) J Heterocyclic Chem 16:1233
a) Caserio MC, Graham WH, Roberts JD (1960) Tetrahedron 11:171; b) Roberts JD, Mazur RH (1951) J Am Chem Soc 73:2509; c) Renk E, Roberts JD (1961) J Am Chem Soc 83:878; Review: Klunder AJH, Zwanenburg B (1997) In: de Meijere A (ed) Houben-Weyl, vol E 17c, Thieme, Stuttgart, p 2419 ff
a) Primke H, Sarin GS, Kohlstruk S, Adiwidjaja G, de Meijere A (1994) Chem Ber 127:1051; b) Primke H (1989) Dissertation, Universität Hamburg
a) Anchel M, Hervey A, Robbins WJ (1950) Proc Nat Acad Sci USA 36:300; b) Anchel M, Hervey A, Robbins WJ (1952) Proc Nat Acad Sci USA 38:927; c) McMorris TC, Anchel M (1965) J Am Chem Soc 87:1594; d) McMorris TC, Kelner MJ, Chadha RK, Siegel JS, Moon S-s, Moya MM (1989) Tetrahedron 45:5433; e) Niwa H, Ojika M, Wakamatsu K, Yamada K, Ohba S, Saito Y, Hirono I, Matsushita K (1983) Tetrahedron Lett 24:5371; f) Ohba S, Saito Y, Hirono I, Niwa H, Ojika M, Wakamatsu K, Yamada K (1984) Acta Cryst C 40:1877; g) Ojika M, Wakamatsu K, Niwa H, Yamada K (1987) Tetrahedron 43:5261; h) Kelner MJ, McMorris TC, Beck WT, Zamora JM, Taetle R (1987) Cancer Med Research 47:3186; i) Kelner MJ, McMorris TC, Taetle R (1990) J Natl Cancer Inst 82:1562; j) McMorris T C, Kelner MJ, Wang W, Moon S, Taetle R (1990) Chem Res Toxicol 3:574; k) McMorris TC, Kelner MJ, Wang W, Estes LA, Montoya MA, Taetle R (1992) J Org Chem 57:6876; l) Review: Yamada K, Ojika M, Kigoshi H (1998) Angew Chem 110:1918 Angew Chem Int Ed Engl 37:1818
Ptaquilosin is the aglycon of the potent carcinogen ptaquiloside, which has the same skeleton as illudin M: a) Kigoshi H, Imamura Y, Mizuta K, Niwa H, Yamada K (1973) J Am Chem Soc 115:3056; b) Kigoshi H, Sawada A, Nakayama Y, Niwa H, Yamada K (1989) Tetrahedron Lett 30:1983; c) Kigoshi H, Imamura Y, Mizuta K, Niwa H, Yamada K (1993) J Am Chem Soc 115:3056. Ptaquiloside: d) Kushida T, Uesugi M, Sugiura Y, Kigoshi H, Tanaka H, Hirokawa J, Ojika M, Yamada K (1994) J Am Chem Soc 116:479; e) Kigoshi H, Imamura Y, Mizuta K, Niwa H, Yamada K (1993) J Am Chem Soc 115:3056; f) Kigoshi H, Tanaka H, Hirokawa J, Mizuta K, Yamada K (1992) Tetrahedron Lett 33:6647
a) Schore NE (1991) Org React 40:1; b) Schore NE (1991) In: Trost BM, Fleming I (eds) Comprehensive Organic Synthesis, Pergamon Press, Oxford, vol 5, p 1037ff; c) Schore NE (1988) Chem Rev 88:1081; d) Pauson PL (1985) Tetrahedron 41:5855; e) Pauson PL (1987) In: de Meijere A, tom Dieck H (eds) Organometallics in Organic Chemistry. Springer, Berlin Heidelberg New York, vol 1, p 233; f) Krafft ME, Chirico X (1994) Tetrahedron Lett 35:4511; g) Oppolzer W, Robyr C (1994) Tetrahedron 50:415
a) Ager DJ, East MB (1993) Tetrahedron 49:5683; b) Vogel P, Fattori D, Gasparini F, Drian CL (1990) Synlett 173
The central carbon atom in methylenecyclopropane is almost sp-hybridized, like that in allene; the carbon atoms in cyclopropane are almost sp 2-hybridized, cf de Meijere A (1979) Angew Chem 91:867 Angew Chem Int Ed Engl 18:809
a) Vill V (1992) Mol Cryst Liq Cryst 213:67; b) Tabohasi T, Sakurai T, Higuchi R, Mikami N, Yamamoto E, Takeuchi K (1987) Eur Pat Appl EP 244, 129CA (1988) 108:P66076j; c) McGaffin G (1993) Dissertation, Universität Hamburg; d) Byron DJ, Matharu AS, Rees M, Wilson RC (1995) Mol Cryst Liq Cryst A258:217
Tamm M (1997) Dissertation, Universität Göttingen
a) O’Donnell MJ, Polt RL (1982) J Org Chem 47:2663; b) O’Donnell MJ, Bennett WD, Polt RL (1985) Tetrahedron Lett 26:695; c) O’Donnell MJ, Falmagne J-B (1985) Tetrahedron Lett 26:699; d) O’Donnell MJ, Zhou C, Mi A, Chen N, Kyle JA, Andersson PG (1995) Tetrahedron Lett 36:4205; e) O’Donnell MJ, Yang X, Li M, Tetrahedron Lett (1990) 31:5135; f) O’Donnell MJ, Bennett WD (1988) Tetrahedron 44:5389; g) O’Donnell MJ, Zhou C, Chen N (1996) Tetrahedron: Asymmetry 7:621
Review: Lipshutz BH, Sengupta S (1992) In: Paquette LA (ed) Org React, Wiley, New York, vol 41 p 135
Tamm M, Thutewohl M, Ricker CB, Bes MT, de Meijere A (1999) Eur J Org Chem:2017
Blandov A, de Meijere A (unpublished results)
Wessjohann L, McGaffin G, de Meijere A (1989) Synthesis 359
Es-Sayed M, Heiner T, de Meijere A (1993) Synlett 57
a) Wessjohann L, Skattebøl L, de Meijere A (1990) J Chem Soc Chem Commun: 574; b) Giller K, Baird MS, de Meijere A (1992) Synlett: 524
a) O’Donnell MJ, Wojciechowski K, Ghosez L, Navarro M, Sainte F, Antoine J-P (1984) Synthesis: 313; b) Stork G, Leong AYW, Touzin AM (1976) J Org Chem 41:3491
Es-Sayed M, Gratkowski C, Krass N, Meyers AI, de Meijere A (1992) Synlett: 962
a) Matsui S, Hashimoto Y, Saito K (1998) Synthesis: 1161; b) Badone D, Bernassau J-M, Cardamone R, Guzzi U (1996) Angew Chem 108:575 Angew Chem Int Ed Engl 35:535; c) Tamura O, Hashimoto M, Kobayashi Y, Katoh T, Nakatani K, Kamada M, Hayakawa I, Akiba T, Terashima S (1992) Tetrahedron Lett 33:3487; d) Newman MS, Kutner A (1951) J Am Chem Soc 73:4199; e) Stefanovsky JN, Spassov SL, Kurtev BJ, Balla M, Ötvös L (1969) Chem Ber 102:717. Other methods of preparation: f) Söderbaum HG (1896) Chem Ber 29:1210; g) Auerbach RA, Kingsbury CA (1971) Tetrahedron 27:2069; h) Hassner A, Burke SS (1974) Tetrahedron 30:2613; i) Okuda M, Tomioka K (1994) Tetrahedron Lett 35:4585; j) Foglia TA, Swern D (1969) J Org Chem 34:1680; k) Pirkle WH, Simmons KA (1983) J Org Chem 48:2520; l) Hoppe D, Follmann R (1976) Chem Ber 109:3047; m) Murthy KS, Keshava DDN (1984) J Heterocycl Chem 21:1721; n) Banks MR, Cadogan JIG, Gosney I, Hodgson PKG, Thomson DE (1991) J Chem Soc Perkin Trans 1:961; o) Murthy KS, Keshava DDN (1984) Synth Commun 14:687; p) Park, YS, Boys ML, Beak P(1996) J Am Chem Soc 118:3757
(S)-4-Phenyloxazolidine-2-one (98), (S)-4-phenyloxazolidine-2-thione (99) and (4R,5S)-4,5-diphenyloxazolidine-2-one (100) are very poor nucleophiles. A substoichiometric amount (10 mol%) of potassium hydride is necessary to generate a fraction of the potassium salt of 13 which has a sufficient nucleophilicity. This holds for the anions generated by deprotonation at the carbamate position and activation by addition of dibenzo-18-crown-6 to the reaction mixture, and causes the Michael addition to be very slow at −78 °C. The best temperature is −20 → 0 °C. The higher the temperature, the more decomposition product is formed during the Michael addition.
a) Duhamel L, Duhamel P, Launay JC, Plaquevent JC (1984) Bull Soc Chim Fr II: 421; b) Potin D, Williams K, Rebek Jr J (1990) Angew Chem 102:1485 Angew Chem Int Ed Engl 29:1420; c) Fehr C, Galindo J (1988) J Am Chem Soc 110:6909
For the analogous reactions which did not get any further development see also ref. [9]
a) Salaün J (1987) In: Rappoport Z (ed) The chemistry of the cyclopropyl group, Wiley, New York, p 809ff; b) Gajewsky JJ, Oberdier JP (1972) J Am Chem Soc 94:6053; c) Erden I, de Meijere A, Rousseau G, Conia JM (1980) Tetrahedron Lett 21:2501; d) Crandall JK, Conower WW (1978) J Org Chem 43:3533; e) Aue DH, Meshishneck MJ, Shellhamer DF (1973) Tetrahedron Lett 4799
a) Aue DH, Lorens RB, Helwig GS (1973) Tetrahedron Lett: 4795; b) Aue DH, Lorens RB, Helwig GS (1979) J Org Chem 44:1202; c) Crandall JK, Conover WW (1973) J Chem Soc Chem Commun 33; d) Crandall JK, Conover WW (1974) J Org Chem 39:63; e) Tsuge O, Ohnishi T, Watanabe H (1981) Heterocycles 16:2085; f) Tsuge O, Watanabe H (1976) Heterocycles 4:1905; g) Tsuge O, Watanabe H (1977) Heterocycles 7:709; h) Tsuge O, Watanabe H, Kiryu Y (1979) Bull Chem Soc Jpn 52:3387; i) Tlili T (1988) J Soc Chim Tunis 2:3
Dauban P, Dubois L, Dau METH, Dodd RH (1995) J Org Chem 60:2035; b) Tanner D (1994) Angew Chem 106:525 Angew Chem Int Ed Engl 33:599 and references cited therein; c) Goodman M (1994) Chemistry and Biology 231
a) Amice P, Conia JM (1974) Bull Soc Chim Fr: 1015; b) Amice P, Conia JM (1974) Tetrahedron Lett: 479; c) Snider BB, Roush DM, Rodini DJ, Gonzalez D, Spindell D (1980) J Org Chem 45:2773; d) Ficini J, Krief A (1970) Tetrahedron Lett: 885; e) Ficini J, Dureault A (1977) Tetrahedron Lett 809
The ring enlargement of a cyclopropylmethylene to a cyclobutene is well known: a) Wiberg KB, Burgmaier GJ, Warner P (1971) J Am Chem Soc 93:246; b) de Meijere A, Wenck H, Kopf J (1988) Tetrahedron 44:2427; c) Ho G-J, Krogh-Jespersen K, Moss RA, Shen S, Sheridan RS, Subramanian R (1989) Am Chem Soc 111:6875
Tamm M, Nötzel M, Noltemeyer M, de Meijere A (2000) J Org Chem (submitted)
Kuchuk I, de Meijere A (unpublished results)
Adachi T, Yamada Y, Inoue I (1977) Synthesis 45
a) Dürckheimer W, Blumbach J, Lattrell R, Scheunemann KH (1985) Angew Chem 97:183 Int Ed Engl 24:180; b) Bell MR, Carlson JA, Oesterlin R (1972) J Org Chem 37:2733; c) Jungheim LN, Sigmund SK, Jones ND, Swartzendruber JK, Lilly E (1987) Tetrahedron Lett 28:289; d) Campbell MM, Connarty BP (1982) Heterocycles 19:1853; e) Campbell MM, Carruthers NI, Mickel SJ, Winton PM (1984) J Chem Soc Chem Commun: 200; f) Barrett AGM, Cheng MC, Spilling CD, Taylor SJ (1989) J Org Chem 54:992
a) Johnson G, Ross BC (1981) J Chem Soc Chem Commun 1269; b) Branch CL, Pearson MJ (1986) J Chem Soc Perkin Trans I: 1077
As for the biological activities of cyclopropane derivatives see: a) Salaün J, S. Baird MS (1995) Curr Med Chem 2:511; b) Salaün J (1999) Top Curr Chem, this volume
Hegedus LS, Montgomery J, Narukawa Y, Snustad DC (1991) J Am Chem Soc 113: 5784
Kirmse W (1965) Angew Chem 77:1 Angew Chem Int Ed Engl 4:1
Review: Korobitsyna IK, Khalikova AV, Rodina LL, Shusherina NP (1983) Khim Geterotsikl Soed 147 Chem Heterocycl Comp (Engl Transl) 117
Breslow R (1963) In: de Mayo P (ed) Molecular rearrangements. Wiley, New York p 233 ff
a) Govindan CK, Taylor G (1983) J Org Chem 48:5348; b) Wendling LA, Bergman RG (1976) J Org Chem 41:831; c) Rossi E, Celentano G, Stradi R, Strada A (1990) Tetrahedron Lett 31:903
a) Boeckman RK Jr, Jackson PF, Sabatucci JP (1985) J Am Chem Soc 107:2191; b) Wasserman HH, Dion RP, Fukuyama J (1989) Tetrahedron 45:3203
a) Málek J (1988) Org React 36:310; b) Hutchins RO, Su W-Y, Sivakumar R, Cistone F, Stercho YP (1983) J Org Chem 48:3412
a) Stevens RV (1977) Acc Chem Res 10:193; b) Boeckman RK Jr, Sabatucci JP, Goldstein SW, Springer DM, Jackson PF (1986) J Org Chem 51:3740; c) Wasserman HH, Fukuyama JM (1991) Tetrahedron Lett 32:7127; d) Maryanoff BE, McComsey DF, Mutter MS, Sorgi KL, Maryanoff CA (1988) Tetrahedron Lett 29:5073
a) Cloke JB (1929) J Am Chem Soc 51:1174; b) Gotkis D, Cloke JB (1934) J Am Chem Soc 56:2710; c) Stevens RV, Ellis MC, Wentland MP (1968) J Am Chem Soc 90:5576
Nötzel M (1999) Part of a forthcoming Dissertation, Universität Göttingen
a) Stacy GW, Day RI, Morath RJ (1955) J Am Chem Soc 77:3869; b) Stacy GW, Craig PA, DayRI (1958) J Org Chem 23:1760; c) Sandhu JS, Sethi PS, Mohan S (1971) J Indian Chem Soc 48:89
Belov VN, Funke C, Labahn T, Es-Sayed M, de Meijere A (1999) Eur J Org Chem 1345
a) Robert M, Barbier M, Lederer E, Roux L, Biemann K, Vetter W (1962) Bull Soc Chim Fr 187; b) Hardegger E, Liechti P, Jackman LM, Boller A, Plattner PA (1963) Helv Chim Acta 46(1):60
a) Burrouhs LF (1957) Nature 179:360; b) Vähätalo M-L, Virtanen AI (1957) Acta Chem Scand 11:741
a) Rosenthal GA (1982) Plant Nonproteinogenic Amino and Imino Acids. Biological and Toxicological Properties, Academic Press, New York; b) Barrett GC (1985) Chemistry and Biochemistry of the Amino Acids, Chapman and Hall, New York, p 64; c) Boller T, Kende H (1980) Nature 286:259; d) Pirrung MC (1987) J Org Chem 52:4179; d) Hill RK, Prakash SR, Wiesendanger R, Angst W, Martinoni B, Arigoni D, Liu H-W, Walsh CT (1984) J Am Chem Soc 106:795; e) Hoffman NE, Yang SF, Ichihara A, Sakamura S (1982) Plant Physiol 70:195
a) Pirrung MC, McGeehan GM (1985) Angew Chem 97:1074 Angew Chem Int Ed Engl 24:1044; b) Mitchel RE, Pirrung MC, McGeehan GM (1987) Phytochemistry 26:2695; c) Ichihara A, Shiraishi K, Sato H, Sakamura S, Nishiyama K, Sakai R, Furusaki A, Matsumoto T (1977) J Am Chem Soc 99:636; d) Bernabe M, Fernadez OC, Fernadez-Alvarez E (1979) Annales de Quimica 75:977; e) Harding WM, DeShazo ML (1967) Arch Biochem Biophys 118:23; f) Johnson RL, Koerner JF (1988) J Med Chem 31:2057; g) Pellicciari R, Natalini B, Marinozzi M, Monahan JB, Snyder JP (1990) Tetrahedron Lett 31:139; h) Stewart FHC (1981) Aust J Chem 34:2431; i) Tsang JW, Schmied B, Nyfeler R, Goodman M (1984) J Med Chem 27:1663; j) Pirrung MC, McGeehan GM (1986) J Org Chem 51:2103; k) Baldwin JE, Adlington RM, Rawlings BJ (1985) Tetrahedron Lett 26:481; l) Pirrung MC, Dunlap SE, Trinks UP (1989) Helv Chim Acta 72:1301; m) Aitken DJ, Royer J, Husson H-P (1990) J Org Chem 55:2814
Stammer CH (1990) Tetrahedron 46:2231; b) Pirrung MC, Cao J, Chen J (1995) J Org Chem 60:5790; c) Burgess K, Lim D, Ho K-K, Ke C-Y (1994) J Org Chem 59:2179
For several recent communications on this topic see: a) Zindel J, de Meijere A (1995) J Org Chem 60:2968; b) Burgess K, Ke C-Y (1996) Synthesis 1463; c) Hercouet A, Bessières B, Le Corre M (1996) Tetrahedron Asymmetry 7:1267; d) Franzone G, Carle S, Dorizon P, Ollivier J, Salaün J (1996) Synlett 1067; e) Hercouet A, Godbert N, Le Corre M (1998) Tetrahedron: Asymmetry 9:2233; f) Fadel A, Khesrani A (1998) Tetrahedron: Asymmetry 9:305; g) Chinchilla R, Falvello LR, Galindo N, Nájera C (1998) Tetrahedron:Asymmetry 9:2223; h) Atlan V, Racouchot S, Rubin M, Bremer C, Ollivier J, de Meijere A, Salaün J (1998) Tetrahedron: Asymmetry 9:1131
a) Baldwin JE, Adlington RM, Bebbington D, Russell AT (1994) Tetrahedron 50:12015 and ref. [2] therein; b) Lai M-t, Liu M-d, Liu H-w (1991) J Am Chem Soc 113:7388; c) Lai M-t, Liu H-w (1992) J Am Chem Soc 114:3160; d) Kurokawa N, Ohfune Y (1985) Tetrahedron Lett 26:83; e) Li K, Du W, Que NLS, Liu H-w (1996) J Am Chem Soc 118:8763
Shoji J-I, Sakazaki R, Kato T, Tori K, Yoshimura Y, Matsuura S (1981) J Antibiotics 34:370
Kato K, Takita T, Umezawa H (1980) Tetrahedron Lett 21:4925
a) Evans DA, Britton TC (1987) J Am Chem Soc 109:6881; b) Evans DA, Ellman JA (1989) J Am Chem Soc 111:1063
a) Aoyagi T, Yoshida S, Matsuda N, Ikeda T, Hamada M, Takeuchi T (1991) J Antibiotics 44:573; b) Angelastro MR, Mehdi S, Burkhart JP, Peet NP, Bey P (1990) J Med Chem 33:13
a) Bunnage ME, Davies SG, Goodwin CJ (1994) J Chem Soc Perkin Trans I: 2385; b) Wang Z-M, Kolb HC, Sharpless KB (1994) J Org Chem 59:5104
a) Katayama N, Nozaki Y, Tsubotani S, Kondo M, Harada S, Ono H (1992) J Antibiotics 45:10; b) Funabashi T, Tsubotani S, Koyama K, Katayama N, Harada S (1993) Tetrahedron 49:13; c) Sokolov VV, Kozhushkov SI, Nikolskaya S, Belov VN, Es-Sayed M, de Meijere A (1998) Eur J Org Chem: 777
Manis PA, Rathke MW (1980) J Org Chem 45:4952
a) Zindel J (1993) Dissertation, Universität Göttingen; b) Gutke H-J (1998) Dissertation, Universität Göttingen
Spitzner D, Engler A, Liese T, Splettstößer G, de Meijere A (1982) Angew Chem 94:799 Angew Chem Int Ed Engl 21:790
Hadjiarapoglou L, de Meijere A, Seitz H-J, Klein I, Spitzner D (1994) Tetrahedron Lett 35:3269
Hadjiarapoglou L, Klein I, Spitzner D, de Meijere A (1996) Synthesis 525
de Meijere A, Hadjiarapoglou LP, Noltemeyer M, Gutke H-J, Klein I, Spitzner D (1998) Eur J Org Chem 441
a) de Meijere A, Kozhushkov SI (1995) In: Halton B (ed) Advances in Strain in Organic Chemistry, JAI Press Ltd., London, vol 4, p 225ff; b) Zefirov NS, Kuznetsova TS, Zefirov AN (1995) Izv Akad Nauk Ser Khim 1613 Russian Chem Bull (Engl Trans) 44:1543
a) Hagiwara H, Kodama T, Kosugi H, Uda H (1976) J Chem Soc Chem Commun 413; b) Gröger C, Musso H (1976) Angew Chem 88:415 Angew Chem Int Ed Engl 15:373
Tsuji T, Kikuchi R, Nishida S (1985) Bull Chem Soc Jpn 58:1603
a) Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT (1971) J Am Chem Soc 93:2325; b) Vidensek N, Lim P, Campell A, Carlson C (1990) J Nat Prod 53:1609; c) Witherup KM, Look SA, Stasko MW, Ghiorzi TJ, Muschik GM, Cragg GM (1990) J Nat Prod 53:1249
a) Swindell CS (1991) Org Prep Procedures Int 23:465; b) Kingston DGI, Molinero AA, Rimoldi JM (1993) Prog Chem Org Nat Prod 61:1; c) Nicolaou KC, Dai W-M, Guy RK (1994) Angew Chem 106:38, Angew Chem Int Ed Engl 33:15; d) Boa AN, Jenkins PR, Lawrence NJ (1994) Contemporary Organic Synthesis 1:47
a) Holton RA, Somoza C, Kim H-B, Liang F, Biediger RJ, Boatman PD, Shindo M, Smith CC, Kim S, Nadizadeh H, Suzuki Y, Tao C, Vu P, Tang S, Zhang P, Murthi KK, Gentile LN, Liu JH (1994) J Am Chem Soc 116:1597; b) Holton RA, Kim H-B, Somoza C, Liang F, Biediger RJ, Boatman PD, Shindo M, Smith CC, Kim S, Nadizadeh H, Suzuki Y, Tao C, Vu P, Tang S, Zhang P, Murthi KK, Gentile LN, Liu JH (1994) J Am Chem Soc 116:1599; c) Nicolaou KC, Yang Z, Liu JJ, Ueno H, Nantermet PG, Guy RK, Claiborne CF, Renaud J, Couladouros EA, Paulvannan K, Sorensen EJ (1994) Nature 367:630
a) Francisco C, Banaigs B, Teste J, Cave A (1986) J Org Chem 51:1115; b) Kakiuchi K, Fukunaga K, Matsuo F, Ohnishi Y, Tobe Y (1991) J Org Chem 56:6742
a) Nagaoka H, Shibuya K, Yamada Y (1993) Tetrahedron Lett 34:1501; b) Nagaoka H, Baba A, Yamada Y (1991) Tetrahedron Lett 32:6741
Wrobel J, Takahashi K, Honkan V, Lannoye G, Cook JM, Bertz SH (1983) J Org Chem 48:139
a) Militzer H-C, Schömenauer S, Otte C, Puls C, Hain J, Bräse S, de Meijere A (1993) Synthesis 998; b) Padwa A, Wannamaker MW (1986) Tetrahedron Lett 27:2555
Gutke H-J, Spitzner D (1999) Tetrahedron 55:3193
For reviews see a) Trost BM (1977) Tetrahedron 33:2615; b) Trost BM (1980) Acc Chem Res 13:385; c) Tsuji J (1980) Organic Synthesis with Palladium Compounds, Springer, Berlin; d) Tsuji J (1986) Tetrahedron 42:4361; e) Tsuji J, Minami I (1987) Acc Chem Res 20:140; f) Consiglio G, Waymouth RM (1989) Chem Rev 89:257; g) Trost BM (1978) Angew Chem 101:1199 Angew Chem Int Ed Engl 28:1173; h) Frost CG, Howarth J, Williams JMJ (1992) Tetrahedron: Asymmetry 3:1089; i) de Meijere A, Meyer FE (1994) Angew Chem 106:2473 Angew Chem Int Ed Engl 33:3279; j) Bräse S, de Meijere A (1998) In: Diederich F, Stang PJ (eds) Metal-catalyzed Cross-coupling Reactions, Wiley-VCH, Weinheim, p 99ff; k) de Meijere A, Bräse S (1991) J Organomet Chem 576:88
a) Ang KH, Bräse S, Steinig AG, Meyer FE, Llebaria A, Voigt K, de Meijere A (1996) Tetrahedron 52:11503; b) Meyer FE, Ang KH, Steinig AG, de Meijere A (1994) Synlett 191
Körbe S, de Meijere A (unpublished results)
Bhat A, Steinig A, Appelbe R, de Meijere A (2000) Eur J Org Chem (to be submitted)
a) Terrett NK, Gardener M, Gordon DW, Kobylecki RJ, Steele J (1995) Tetrahedron 51:8135; b) Balkenhohl F, Bussche-Hünnefeld C, Lansky A, Zechel C (1996) Angew Chem 108:2436 Angew Chem Int Ed Engl 35:2288; c) Nefzi A, Ostresh JM, Houghten RA (1997) Chem Rev 97:449; d) Szardenings AK, Burkoth TS, Lu HH, Tien DW, Campbell DA (1997) Tetrahedron 53:6573; e) Dömling A (1998) Comb Chem & High Troughput Screen 1:1
Terrett NK (1998) Combinatorial Chemistry, Oxford University Press, Oxford
Khlebnikov AF, de Meijere A (unpublished results)
Hughes DL (1992) In: Paquette L (ed) Org React, Wiley, New York, vol 42, p 335
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2000 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
de Meijere, A., Kozhushkov, S.I., Hadjiarapoglou, L.P. (2000). Alkyl 2-Chloro-2-cyclopropylideneacetates—Remarkably Versatile Building Blocks for Organic Synthesis. In: de Meijere, A. (eds) Small Ring Compounds in Organic Synthesis VI. Topics in Current Chemistry, vol 207. Springer, Berlin, Heidelberg. https://doi.org/10.1007/3-540-48255-5_4
Download citation
DOI: https://doi.org/10.1007/3-540-48255-5_4
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-540-66471-0
Online ISBN: 978-3-540-48255-0
eBook Packages: Springer Book Archive