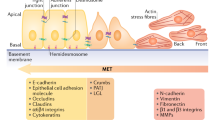
Abstract
The epithelial to mesenchymal transition (EMT) is characterized by the loss of epithelial characteristics and the gain of mesenchymal attributes in epithelial cells. It has been associated with physiological and pathological processes requiring epithelial cell migration and invasion. Initially, EMT was observed in embryological and adult development with many well characterized examples including the conversions of epiblast to primary mesenchyme (gastrulation), somite to sderotome, somite to dermis, myotome to migratory myoblast, dorsal neural tube to neural crest, placodal ectoderm to cranial ganglion precursor, intermediate mesoderm to nephric mesenchyme, lateral mesoderm to connective/muscular tissue, endocardium to cardiac cushion mesenchyme and trophectoderm invasion.[1],[2] In addition, evidence is mounting to support an important role of EMT pathways in the progression of carcinoma to metastasis providing epithelial tumour cells with the ability to migrate, invade the surrounding stroma and disseminate in secondary organs.[3]–[5]
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Hay ED, Zuk A. Transformations between epithelium and mesenchyme: normal, pathological, and experimentally induced. Am J Kidney Dis 1995; 26(4):678–690.
Duband JL, Monier F, Delannet M et al. Epithelium-mesenchyme transition during neural crest development. Acta Anat 1995; 154(1):63–78.
Birchmeier C, Birchmeier W, Brand-Saberi B. Epithelial-mesenchymal transitions in cancer progression. Acta Anat 1996; 156(3):217–226.
Thiery JP. Epithelial-mesenchymal transitions in development and pathologies. Curr Opin Cell Biol 2003; 15(6):740–746.
Gotzmann J, Mikula M, Eger A et al. Molecular aspects of epithelial cell plasticity: implications for local tumour invasion and metastasis. Mutat Res 2004; 566(1):9–20.
Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat 1995; 154(1):8–20.
Viebahn C, Lane EB, Ramaekers FC. Keratin and vimentin expression in early organogenesis of the rabbit embryo. Cell Tissue Res 1988; 253(3):553–562.
Erickson CA, Tucker RP, Edwards BF. Changes in the distribution of intermediate-filament types in Japanese quail embryos during morphogenesis. Differentiation 1987; 34(2):88–97.
Boyer B, Valles AM, Edme N. Induction and regulation of epithelial-mesenchymal transitions. Biochem Pharmacol 2000; 60(8):1091–1099.
Reichert M, Muller T, Hunziker W. The PDZ domains of zonula occludens-1 induce an epithelial to mesenchymal transition of Madin-Darby canine kidney I cells. Evidence for a role of beta-catenin/Tcf/Lef signaling. J Biol Chem 2000; 275(13):9492–9500.
Tucker GC, Boyer B, Gavrilovic J et al. Collagen-mediated dispersion of NBT-II rat bladder carcinoma cells. Cancer Res 1990; 50(1):129–137.
Lochter A, Galosy S, Muschler J et al. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J Cell Biol 1997; 139:1861–1872.
Batlle E, Sancho E, Franci C et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2000; 2(2):84–89.
Cano A, Perez-Moreno MA, Rodrigo I et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2000; 2(2):76–83.
Savagner P, Yamada KM, Thiery JP. The zinc-finger protein slug causes desmosome dissociation, an initial and necessary step for growth factor-induced epithelial-mesenchymal transition. J Cell Biol 1997; 137(6):1403–1419.
Savagner P. Leaving the neighborhood: molecular mechanisms involved during epithelial-mesenchymal transition. Bioessays 2001; 23(10):912–923.
Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol 2002; 3(3):155–166.
Oikawa T, Yamada T. Molecular biology of the Ets family of transcription factors. Gene 2003; 303:11–34.
Comijn J, Berx G, Vermassen P et al. The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell 2001; 7(6):1267–1278.
Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta 2003; 1653(1):1–24.
Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 2001; 17:463–516.
Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer 2002; 2(3):161–174.
Benaud C, Dickson RB, Thompson EW. Roles of the matrix metalloproteinases in mammary gland development and cancer. Breast Cancer Res Treat 1998; 50(2):97–116.
Lafleur MA, Handsley MM, Edwards DR. Metalloproteinases and their inhibitors in angiogenesis. Expert Rev Mol Med 2003; 5:1–39.
Noe V, Fingleton B, Jacobs K et al. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. J Cell Sci 2001; H4 (Pt 1):111–118.
Kajita M, Itoh Y, Chiba T et al. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol 2001; 153(5):893–904.
Deryugina EI, Ratnikov B, Monosov E et al. MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res 2001; 263(2):209–223.
Codony-Servat J, Albanell J, Lopez-Talavera JC et al. Cleavage of the HER2 ectodomain is a pervanadate-activable process that is inhibited by the tissue inhibitor of metalloproteases-1 in breast cancer cells. Cancer Res 1999; 59(6):1196–1201.
Vecchi M, Rudolph-Owen LA, Brown CL et al. Tyrosine phosphorylation and proteolysis. Pervanadate-induced, metalloprotease-dependent cleavage of the ErbB-4 receptor and amphiregulin. J Biol Chem 1998; 273(32):20589–20595.
Nath D, Williamson NJ, Jarvis R et al. Shedding of c-Met is regulated by crosstalk between a G-protein coupled receptor and the EGF receptor and is mediated by a TIMP-3 sensitive metalloproteinase. J Cell Sci 2001; H4 (Pt 6):1213–1220.
Barsky SH, Siegal GP, Jannotta F et al. Loss of basement membrane components by invasive tumours but not by their benign counterparts. Lab Invest 1983; 49(2):140–147.
Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer 1997; 80(8 Suppl):1529–1537.
Nelson AR, Fingleton B, Rothenberg ML et al. Matrix Metalloproteinases: Biologic Activity and Clinical Implications. J Clin Oncol 2000; 18(5):1135.
Zucker S, Pei D, Cao J et al. Membrane type-matrix metalloproteinases (MT-MMP). Curr Top Dev Biol 2003; 54:1–74.
English WR, Puente XS, Freije JM et al. Membrane type 4 matrix metalloproteinase (MMP17) has tumour necrosis factor-alpha convertase activity but does not activate pro-MMP2. J Biol Chem 2000; 275(19):14046–14055.
Sato H, Takino T, Okada Y et al. A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 1994; 370(6484):61–65.
Seiki M, Mori H, Kajita M et al. Membrane-type 1 matrix metalloproteinase and cell migration. Biochem Soc Symp 2003 (70):253–262.
Hotary K, Allen E, Punturieri A et al. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J Cell Biol 2000; 149(6):1309–1323.
Wilson CL, Matrisian LM. Matrilysin: an epithelial matrix metalloproteinase with potentially novel functions. Int J Biochem Cell Biol 1996; 28(2):123–136.
Almholt K, Johnsen M. Stromal cell involvement in cancer. Recent Results Cancer Res 2003; 162:31–42.
Janda E, Lehmann K, Killisch I et al. Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: dissection of Ras signaling pathways. J Cell Biol 2002; 156(2):299–313.
Nabeshima K, Inoue T, Shimao Y et al. Matrix metalloproteinases in tumour invasion: role for cell migration. Pathol Int 2002; 52(4):255–264.
Kulesa P, Ellies DL, Trainor PA. Comparative analysis of neural crest cell death, migration, and function during vertebrate embryogenesis. Dev Dyn 2004; 229(1):14–29.
Savagner P, Valles AM, Jouanneau J et al. Alternative splicing in fibroblast growth factor receptor 2 is associated with induced epithelial-mesenchymal transition in rat bladder carcinoma cells. Mol Biol Cell 1994; 5(8):851–862.
Gavrilovic J, Moens G, Thiery JP et al. Expression of transfected transforming growth factor alpha induces a motile fibroblast-like phenotype with extracellular matrix-degrading potential in a rat bladder carcinoma cell line. Cell Regul 1990; 1(13):1003–1014.
Jouanneau J, Gavrilovic J, Caruelle D et al. Secreted or nonsecreted forms of acidic fibroblast growth factor produced by transfected epithelial cells influence cell morphology, motility, and in vasive potential. Proc Natl Acad Sci USA 1991; 88(7):2893–2897.
Bae SN, Arand G, Azzam H et al. Molecular and cellular analysis of basement membrane invasion by human breast cancer cells in Matrigel-based in vitro assays. Breast Cancer Res Treat 1993; 24(3):241–255.
Gilles CT. The epithelial to mesenchymal transition and metastatic progression in carcinoma. The Breast J 1996; 2(2):83–96.
Sommers CL, Thompson EW, Torri JA et al. Cell adhesion molecule uvomorulin expression in human breast cancer cell lines: relationship to morphology and invasive capacities. Cell Growth Differ 1991; 2(8):365–372.
Sommers CL, Heckford SE, Skerker JM et al. Loss of epithelial markers and acquisition of vimentin expression in adriamycin-and vinblastine-resistant human breast cancer cell lines. Cancer Res 1992; 52(19):5190–5197.
Thompson EW, Paik S, Brunner N et al. Association of increased basement membrane invasive-ness with absence of estrogen receptor and expression of vimentin in human breast cancer cell lines. J Cell Physiol 1992; 150(3):534–544.
Sommers CL, Byers SW, Thompson EW et al. Differentiation state and invasiveness of human breast cancer cell lines. Breast Cancer Res Treat 1994; 31(2–3):325–335.
Nawrocki Raby B, Polette M, Gilles C et al. Quantitative cell dispersion analysis: new test to measure tumour cell aggressiveness. Int J Cancer 2001; 93(5):644–652.
Gilles C, Polette M, Birembaut P et al. Expression of c-ets-1 mRNA is associated with an invasive, EMT-derived phenotype in breast carcinoma cell lines. Clin Exp Metastasis 1997; 15(5):519–526.
Azzam HS, Arand G, Lippman ME et al. Association of MMP-2 activation potential with metastatic progression in human breast cancer cell lines independent of MMP-2 production. J Natl Cancer Inst 1993; 85(21):1758–1764.
Pulyaeva H, Bueno J, Polette M et al. MT1-MMP correlates with MMP-2 activation potential seen after epithelial to mesenchymal transition in human breast carcinoma cells [published erratum appears in Clin Exp Metastasis 1997; 15(3):338]. Clin Exp Metastasis 1997; 15(2):111–120.
Gilles C, Polette M, Seiki M et al. Implication of collagen type I-induced membrane-type 1-matrix metalloproteinase expression and matrix metalloproteinase-2 activation in the metastatic progression of breast carcinoma. Lab Invest 1997; 76:651–660.
Giambernardi TA, Grant GM, Taylor GP et al. Overview of matrix metalloproteinase expression in cultured human cells. Matrix Biol 1998; 16(8):483–496.
Grant GM, Giambernardi TA, Grant AM et al. Overview of expression of matrix metalloproteinases (MMP-17, MMP-18, and MMP-20) in cultured human cells. Matrix Biol 1999; 18(2):145–148.
Zajchowski DA, Bartholdi MF, Gong Y et al. Identification of gene expression profiles that predict the aggressive behavior of breast cancer cells. Cancer Res 2001; 61(13):5168–5178.
Jechlinger M, Grunert S, Tamir IH et al. Expression profiling of epithelial plasticity in tumour progression. Oncogene 2003; 22(46):7155–7169.
Thompson EW, Torri J, Sabol M et al. Oncogene-induced basement membrane invasiveness in human mammary epithelial cells. Clin Exp Metastasis 1994; 12(3):181–194.
Giunciuglio D, Culty M, Fassina G et al. Invasive phenotype of MCF10A cells overexpressing c-Ha-ras and c-erbB-2 oncogenes. Int J Cancer 1995; 63(6):815–822.
Gilles C, Polette M, Zahm J et al. Vimentin contributes to human mammary epithelial cell migration [In Process Citation]. J Cell Sci 1999; 112 (Pt 24):4615–4625.
Gilles C, Polette M, Mestdagt M et al. Transactivation of vimentin by beta-catenin in human breast cancer cells. Cancer Res 2003; 63(10):2658–2664.
Gilles C, Polette M, Coraux C et al. Contribution of MT1-MMP and of human laminin-5 gamma2 chain degradation to mammary epithelial cell migration. J Cell Sci 2001; 114 (Pt l6):2967–2976.
Sternlicht MD, Lochter A, Sympson CJ et al. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell 1999; 98(2): 137–146.
Uria JA, Werb Z. Matrix metalloproteinases and their expression in mammary gland. Cell Res 1998; 8(3):187–194.
Witty JP, Wright JH, Matrisian LM. Matrix metalloproteinases are expressed during ductal and alveolar mammary morphogenesis, and misregulation of stromelysin-1 in transgenic mice induces unscheduled alveolar development. Mol Biol Cell 1995; 6(10):1287–1303.
Sternlicht MD, Bissell MJ, Werb Z. The matrix metalloproteinase stromelysin-1 acts as a natural mammary tumour promoter. Oncogene 2000; 19(8):1102–1113.
Lochter A, Srebrow A, Sympson CJ et al. Misregulation of stromelysin-1 expression in mouse mammary tumour cells accompanies acquisition of stromelysin-1-dependent invasive properties. J Biol Chem 1997; 272(8):5007–5015.
Lochter A, Werb Z, Bissell MJ. Transcriptional regulation of stromelysin-1 gene expression is altered during progression of mouse mammary epithelial cells from functionally normal to malignant. Matrix Biol 1999; 18(5):455–467.
Heppner KJ, Matrisian LM, Jensen RA et al. Expression of most matrix metalloproteinase family members in breast cancer represents a tumour-induced host response. Am J Pathol 1996; l49(1):273–282.
Miettinen PJ, Ebner R, Lopez AR et al. TGF-beta induced transdifferentiation of mammary epithelial cells to mesenchymal cells: involvement of type I receptors. J Cell Biol 1994; 127(6 Pt 2):2021–2036.
Piek E, Moustakas A, Kurisaki A et al. TGF-β type I receptor/ALK-5 and smad proteins mediate epithelial to mesenchymal transdifferentiation in NMuMG breast epithelial cells [In Process Cita tion]. J Cell Sci 1999; 112 (Pt 24):4557–4568.
Lelekakis M, Moseley JM, Martin TJ et al. A novel orthotopic model of breast cancer metastasis to bone. Clin Exp Metastasis 1999; 17(2): 163–170.
Tester AM, Ruangpanit N, Anderson RL et al. MMP-9 secretion and MMP-2 activation distinguish invasive and metastatic sublines of a mouse mammary carcinoma system showing epithelial-mesenchymal transition traits. Clin Exp Metastasis 2000; 18(7):553–560.
Martorana AM, Zheng G, Crowe TC et al. Epithelial cells up-regulate matrix metalloproteinases in cells within the same mammary carcinoma that have undergone an epithelial-mesenchymal transition. Cancer Res 1998; 58(21):4970–4979.
Polette M, Gilles C, de Bentzmann S et al. Association of fibroblastoid features with the invasive phenotype in human bronchial cancer cell lines. Clin Exp Metastasis 1998; 16(2):105–112.
Nawrocki-Raby B, Gilles C, Polette M et al. E-Cadherin mediates MMP down-regulation in highly invasive bronchial tumour cells. Am J Pathol 2003; 163(2):653–661.
Jung M, Romer A, Keyszer G et al. mRNA expression of the five membrane-type matrix metalloproteinases MT1-MT5 in human prostatic cell lines and their down-regulation in human malignant prostatic tissue. Prostate 2003; 55(2):89–98.
Daja MM, Niu X, Zhao Z et al. Characterization of expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in prostate cancer cell lines. Prostate Cancer Prostatic Dis 2003; 6(1):15–26.
Gilles C, Piette J, Peter W et al. Differentiation ability and oncogenic potential of HPV-33-and HPV-33 + ras-transfected keratinocytes. Int J Cancer 1994; 58(6):847–854.
Gilles C, Polette M, Piette J et al. Epithelial-to-mesenchymal transition in HPV-33-transfected cervical keratinocytes is associated with increased invasiveness and expression of gelatinase A. Int J Cancer 1994; 59(5):661–666.
Yokoyama K, Kamata N, Fujimoto R et al. Increased invasion and matrix metalloproteinase-2 expression by Snail-induced mesenchymal transition in squamous cell carcinomas. Int J Oncol Apr 2003; 22(4):891–898.
Polette M, Nawrocki-Raby B, Gilles C et al. Tumour Invasion and Matrix Metalloproteases. 2004; In press.
Gilles C, Polette M, Piette J et al. High level of MT-MMP expression is associated with invasiveness of cervical cancer cells. Int J Cancer 1996; 65(2):209–213.
Polette M, Nawrocki B, Gilles C et al. MT-MMP expression and localisation in human lung and breast cancers. Virchows Arch 1996; 428(1):29–35.
Yamamura T, Nakanishi K, Hiroi S et al. Expression of membrane-type-1-matrix metalloproteinase and metalloproteinase-2 in nonsmall cell lung carcinomas. Lung Cancer 2002; 35(3):249–255.
Trudel D, Fradet Y, Meyer F et al. Significance of MMP-2 expression in prostate cancer: an immunohistochemical study. Cancer Res 2003; 63(23):8511–8515.
Kikuchi R, Noguchi T, Takeno S et al. Immunohistochemical detection of membrane-type-1-matrix metalloproteinase in colorectal carcinoma. Br J Cancer 2000; 83(2):215–218.
Ohtani H, Motohashi H, Sato H et al. Dual over-expression pattern of membrane-type metalloproteinase-1 in cancer and stromal cells in human gastrointestinal carcinoma revealed by in situ hybridization and immunoelectron microscopy. Int J Cancer 1996; 68(5):565–570.
Etoh T, Inoue H, Yoshikawa Y et al. Increased expression of collagenase-3 (MMP-13) and MT1-MMP in oesophageal cancer is related to cancer aggressiveness. Gut 2000; 47(1):50–56.
Ohashi K, Nemoto T, Nakamura K et al. Increased expression of matrix metalloproteinase 7 and 9 and membrane type 1-matrix metalloproteinase in esophageal squamous cell carcinomas. Cancer 2000; 88(10):2201–2209.
Cazorla M, Hernandez L, Nadal A et al. Collagenase-3 expression is associated with advanced local invasion in human squamous cell carcinomas of the larynx. J Pathol 1998; 186(2):144–150.
Ueno H, Nakamura H, Inoue M et al. Expression and tissue localization of membrane-types 1, 2, and 3 matrix metalloproteinases in human invasive breast carcinomas. Cancer Res 1997; 57(10):2055–2060.
Ishigaki S, Toi M, Ueno T et al. Significance of membrane type 1 matrix metalloproteinase ex pression in breast cancer. Jpn J Cancer Res 1999; 90(5):516–522.
Nakamura H, Ueno H, Yamashita K et al. Enhanced production and activation of progelatinase A mediated by membrane-type 1 matrix metalloproteinase in human papillary thyroid carcinomas. Cancer Res 1999; 59(2):467–473.
Davidson B, Goldberg I, Gotlieb WH et al. High levels of MMP-2, MMP-9, MT1-MMP and TIMP-2 mRNA correlate with poor survival in ovarian carcinoma. Clin Exp Metastasis 1999; 17(10):799–808.
Davidson B, Goldberg I, Berner A et al. Expression of membrane-type 1, 2, and 3 matrix metalloproteinases messenger RNA in ovarian carcinoma cells in serous effusions. Am J Clin Pathol 2001; 115(4):517–524.
Imanishi Y, Fujii M, Tokumaru Y et al. Clinical significance of expression of membrane type 1 matrix metalloproteinase and matrix metalloproteinase-2 in human head and neck squamous cell carcinoma. Hum Pathol 2000; 31(8):895–904.
Maatta M, Soini Y, Liakka A et al. Differential expression of matrix metalloproteinase (MMP)-2, MMP-9, and membrane type 1-MMP in hepatocellular and pancreatic adenocarcinoma: implications for tumour progression and clinical prognosis. Clin Cancer Res 2000; 6(7):2726–2734.
Ahmad A, Hanby A, Dublin E et al. Stromelysin 3: an independent prognostic factor for relapse-free survival in node-positive breast cancer and demonstration of novel breast carcinoma cell expression. Am J Pathol 1998; 152(3):721–728.
Wright JH, McDonnell S, Portella G, et al. A switch from stromal to tumour cell expression of stromelysin-1 mrna associated with the conversion of squamous to spindle carcinomas during mouse skin tumour progression. Mol Carcinog 1994; 10(4):207–215.
Kadono Y, Shibahara K, Namiki M et al. Membrane type 1-matrix metalloproteinase is involved in the formation of hepatocyte growth factor/scatter factor-induced branching tubules in madin-darby canine kidney epithelial cells. Biochem Biophys Res Commun 1998; 251(3):681–687.
Kadono Y, Okada Y, Namiki M et al. Transformation of epithelial Madin-Darby canine kidney cells with p60(v-src) induces expression of membrane-type 1 matrix metalloproteinase and invasiveness. Cancer Res 1998; 58(10):2240–2244.
Noritake H, Miyamori H, Goto C et al. Overexpression of tissue inhibitor of matrix metalloproteinases-1 (TIMP-1) in metastatic MDCK cells transformed by v-src. Clin Exp Metastasis. 1999; 17(2):105–110.
Miyamori H, Hasegawa K, Kim KR et al. Expression of metastasis-associated mts1 gene is co-induced with membrane type-1 matrix metalloproteinase (MT1-MMP) during oncogenic transformation and tubular formation of Madin Darby canine kidney (MDCK) epithelial cells. Clin Exp Metastasis 2000; 18(1):51–56.
Lelongt B, Trugnan G, Murphy G et al. Matrix metalloproteinases MMP2 and MMP9 are produced in early stages of kidney morphogenesis but only MMP9 is required for renal organogenesis in vitro. J Cell Biol 1997; 136(6):1363–1373.
Li Y, Yang J, Dai C et al. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest 2003; 112(4):503–516.
Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest 2003; 112(12):1776–1784.
Cheng S, Lovett DH. Gelatinase A (MMP-2) is necessary and sufficient for renal tubular cell epithelial-mesenchymal transformation. Am J Pathol 2003; 162(6):1937–1949.
Buisson AC, Zahm JM, Polette M et al. Gelatinase B is involved in the in vitro wound repair of human respiratory epithelium. J Cell Physiol 1996; 166(2):413–426.
Legrand C, Gilles C, Zahm JM et al. Airway epithelial cell migration dynamics. MMP-9 role in cell-extracellular matrix remodeling. J Cell Biol 1999; 146(2):517–529.
Buisson AC, Gilles C, Polette M et al. Wound repair-induced expression of a stromelysins is associated with the acquisition of a mesenchymal phenotype in human respiratory epithelial cells. Lab Invest 1996; 74(3):658–669.
Markwald R, Eisenberg C, Eisenberg L et al. Epithelial-mesenchymal transformations in early avian heart development. Acta Anat 1996; 156(3):173–186.
Alexander SM, Jackson KJ, Bushnell KM et al. Spatial and temporal expression of the 72-kDa type IV collagenase (MMP-2) correlates with development and differentiation of valves in the embryonic avian heart. Dev Dyn 1997; 209(3):261–268.
Cai DH, Vollberg TM Sr., Hahn-Dantona E et al. MMP-2 expression during early avian cardiac and neural crest morphogenesis. Anat Rec 2000; 259(2):168–179.
Duong TD, Erickson CA. MMP-2 plays an essential role in producing epithelial-mesenchymal transformations in the avian embryo. Dev Dyn 2004; 229(1):42–53.
Song W, Jackson K, McGuire PG. Degradation of type IV collagen by matrix metalloproteinases is an important step in the epithelial-mesenchymal transformation of the endocardial cushions. Dev Biol 2000; 227(2):606–617.
Macias D, Perez-Pomares JM, Garcia-Garrido L et al. Immunoreactivity of the ets-1 transcription factor correlates with areas of epithelial-mesenchymal transition in the develo** avian heart. Anat Embryol (Berl) 1998; 198(4):307–315.
Carmona R, Gonzalez-Iriarte M, Macias D et al. Immunolocalization of the transcription factor Slug in the develo** avian heart. Anat Embryol (Berl) 2000; 201(2):103–109.
Vicovac L, Aplin JD. Epithelial-mesenchymal transition during trophoblast differentiation. Acta Anat 1996; 156(3):202–216.
Nawrocki B PM, Maquoi E, Birembaut P. Expression of matrix metalloproteinases and their inhibitors during human placental development. Trophoblast Research 1997; 10:97–113.
Nawrocki B, Polette M, Marchand V et al. Membrane-type matrix metalloproteinase-1 expression at the site of human placentation. Placenta 1996; 17(8):565–572.
Polette M, Nawrocki B, Pintiaux A et al. Expression of gelatinases A and B and their tissue inhibitors by cells of early and term human placenta and gestational endometrium. Lab Invest 1994; 71(6):838–846.
Librach CL, Werb Z, Fitzgerald ML et al. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol 1991; 113(2):437–449.
del Barrio MG, Nieto MA. Overexpression of Snail family members highlights their ability to promote chick neural crest formation. Development 2002; 129(7):1583–1593.
Fafeur V, Tulasne D, Queva C et al. The ETS1 transcription factor is expressed during epithelial-mesenchymal transitions in the chick embryo and is activated in scatter factor-stimulated MDCK epithelial cells. Cell Growth Differ 1997; 8(6):655–665.
Giambernardi TA, Sakaguchi AY, Gluhak J et al. Neutrophil collagenase (MMP-8) is expressed during early development in neural crest cells as well as in adult melanoma cells. Matrix Biol 2001; 20(8):577–587.
Cai DH, Brauer PR. Synthetic matrix metalloproteinase inhibitor decreases early cardiac neural crest migration in chicken embryos. Dev Dyn 2002; 224(4):441–449.
Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumour invasion. Faseb J May 1999; 13(8):781–792.
Singh S, Barrett J, Sakata K et al. ETS proteins and MMPs: partners in invasion and metastasis. Curr Drug Targets 2002; 3(5):359–367.
Brabletz T, Jung A, Dag S et al. beta-catenin regulates the expression of the matrix metalloproteinase-7 in human colorectal cancer. Am J Pathol 1999; 155(4):1033–1038.
Crawford HC, Fingleton BM, Rudolph-Owen LA et al. The metalloproteinase matrilysin is a tar get of beta-catenin transactivation in intestinal tumours. Oncogene 1999; 18(18):2883–2891.
Crawford HC, Fingleton B, Gustavson MD et al. The PEA3 subfamily of Ets transcription factors synergizes with beta-catenin-LEF-1 to activate matrilysin transcription in intestinal tumours. Mol Cell Biol 2001; 21(4):1370–1383.
Takahashi M, Tsunoda T, Seiki M et al. Identification of membrane-type matrix metalloproteinase-1 as a target of the beta-catenin/Tcf4 complex in human colorectal cancers. Oncogene 2002;21(38):5861–5867.
Marchenko GN, Marchenko ND, Leng J et al. Promoter characterization of the novel human matrix metalloproteinase-26 gene: regulation by the T-cell factor-4 implies specific expression of the gene in cancer cells of epithelial origin. Biochem J 2002; 363 (Pt 2):253–262.
Coussens LM, Fingleton B, Matrisian LM. Matrix metalloproteinase inhibitors and cancer: trials and tribulations. Science 2002; 295(5564):2387–2392.
Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer 2002; 2(9):657–672.
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2005 Eurekah.com and Kluwer Academic / Plenum Publishers
About this chapter
Cite this chapter
Gilles, C., Newgreen, D.F., Sato, H., Thompson, E.W. (2005). Matrix Metalloproteases and Epithelial-to-Mesenchymal Transition. In: Rise and Fall of Epithelial Phenotype. Molecular Biology Intelligence Unit. Springer, Boston, MA. https://doi.org/10.1007/0-387-28671-3_20
Download citation
DOI: https://doi.org/10.1007/0-387-28671-3_20
Publisher Name: Springer, Boston, MA
Print ISBN: 978-0-306-48239-7
Online ISBN: 978-0-387-28671-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)