Abstract
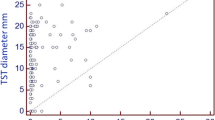
Problem considered: Working in hospital wards, especially the infectious and pulmonary diseases wards, increases the likelihood of the risk of the health care staff with a variety of infectious factors, including the Mycobacterium tuberculosis. The present study was aimed to evaluate the QFT and TST test as well as vitamin D deficiency for screening the tuberculosis health care staff of the tuberculosis sections in Khorasan Razavi province. Methods: In this respect, 44 Tuberculosis health care staff of the tuberculosis sections in Khorasan Razavi province who are directly related to tuberculosis patients were included. TST was performed and chest radiography was done on all the subjects on the day of sampling and the results were collected. The expression of T-bet, FOXP3, and RORyt genes was evaluated by TaqMan Real-time PCR after the lymphocyte isolation by gradient Ficoll lymphocyte assay for immunological isolation and after cDNA synthesis. QFT was performed using the QFT-plus kit the day after sampling in accordance with the instructions in the kit. Results: Out of the 54 TB staff in the province, who were invited for the test, 16 personnel refused to follow the test. Out of 38 participants, TST was positive for 6 people (15.7%), and the QFT test was also positive for 10 people (26.3%), while 4 (10.5%) people showed positive results in both tests. Among 38 cases, the agreement between the two TST and QFT tests was 63% (P-value < 0.0001) among the 38 subjects. The sensitivity and specificity of the TST test were 40 and 93%, respectively as compared to those of QFT. Comparing the results of the QFT positive group to the QFT negative group, the T-bet gene expression in the QFT positive group was 130.9 and the QFT negative group was 34.06, which were statistically significant. Thus, the expression of this gene in the QFT positive group was 3.8 times higher than that of the negative QFT group. Moreover, Foxp3 gene expression in the QFT group was equivalent to 233.3, and in the negative QFT, the group was equivalent to 93.5, which was statistically significant. Therefore, the expression of this gene in the QFT positive group showed an increase 2.3 times higher than that of the negative QFT group. Conclusion: According to the results of this study, the QFT test can be an appropriate alternative for skin tests in a high-risk group for TB.

Similar content being viewed by others
REFERENCES
Ombura, I.P.O., Odera, N., Mutua, S., and Nyagol, F.J., Prevalence of drug resistance Mycobacterium tuberculosis among patients seen in coast provincial general hospital, Mombasa, Kenya, PLoS One, 2016, vol. 11, no. 10, article no. e0163994.
Global Tuberculosis Report 2019, Geneva: World Health Organization, 2019.
Marks, G.B., Bai, J., Simpson, S.E., Sullivan, E.A., and Stewart, G.J., Incidence of tuberculosis among a cohort of tuberculin-positive refugees in Australia: reappraising the estimates of risk, Am. J. Respir. Crit. Care Med., 2000, vol. 162, no. 5, pp. 1851–1854.
Jensen, P.A., Lambert, L.A., Iademarco, M.F., and Ridzon, R., Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Health-Care Settings, Center for Disease Control and Prevention, 2005.
Zwerling, A., van den Hof, S., and Scholten, J., Interferon-gamma release assays for tuberculosis screening of healthcare workers: A systematic review, Thorax, 2011, vol. 67, no. 1, pp. 62–70.
Ringshausen, F.C., Schablon, A., and Nienhaus, A., Interferon-gamma release assays for the tuberculosis serial testing of health care workers: A systematic review, J. Occup. Med. Toxicol., 2012, vol. 7, no. 1, p. 6.
Turner, J. and Dockrell, H., Stimulation of human peripheral blood mononuclear cells with live Mycobacterium bovis BCG activates cytolytic CD8+ T cells in vitro, Immunology, 1996, vol. 87, no. 3, pp. 339–342.
Busch, M., Herzmann, C., Kallert, S., Zimmermann, A., Höfer, C., Mayer, D., et al., Lipoarabinomannan-responsive polycytotoxic T cells are associated with protection in human tuberculosis, Am. J. Respir. Crit. Care Med., 2016, vol. 194, no. 3, pp. 345–355.
Brookes, R.H., Pathan, A.A., McShane, H., Hensmann, M., Price, D.A., and Hill, A.V., CD8 T cell-mediated suppression of intracellular Mycobacterium tuberculosis growth in activated human macrophages, Eur. J. Immunol., 2003, vol. 33, no. 12, pp. 3293–3302.
Pai, M., Denkinger, C.M., Kik, S.V., Rangaka, M.X., Zwerling, A., Oxlade, O., et al., Gamma interferon release assays for detection of Mycobacterium tuberculosis infection, Clin. Microbiol. Rev., 2014, vol. 27, no. 1, pp. 3–20.
Menzies, D., Joshi, R., and Pai, M., Risk of tuberculosis infection and disease associated with work in health care settings, Int. J. Tuberc. Lung Dis., 2007, vol. 11, no. 6, pp. 593–605.
Holick, M.F., High prevalence of vitamin D inadequacy and implications for health, Mayo Clin. Proc., 2006, vol. 81, no. 3, pp. 353–373.
Wilkinson, R.J., Llewelyn, M., Toossi, Z., Patel, P., Pasvol, G., Lalvani, A., et al., Influence of vitamin d deficiency and vitamin D receptor polymorphisms on tuberculosis among Gujarati Asians in west, London: A case-control study, Lancet, 2000, vol. 355, no. 9204, pp. 618–621.
Talat, N., Perry, S., Parsonnet, J., Dawood, G., and Hussain, R., Vitamin D deficiency and tuberculosis progression, Emerging Infect. Dis., 2010, vol. 16, no. 5, p. 853.
Sita-Lumsden, A., Lapthorn, G., Swaminathan, R., and Milburn, H.J., Reactivation of tuberculosis and vitamin D deficiency: the contribution of diet and exposure to sunlight, Thorax, 2007, vol. 62, no. 11, pp. 1003–1007.
Nnoaham, K.E. and Clarke, A., Low serum vitamin D levels and tuberculosis: A systematic review and meta-analysis, Int. J. Epidemiol., 2008, vol. 37, no. 1, pp. 113–119.
Lemire, J.M., Immunomodulatory role of 1, 25-dihydroxyvitamin D3, J. Cell. Biochem., 1992, vol. 49, no. 1, pp. 26–31.
Reichel, H., Koeffler, H.P., Tobler, A., and Norman, A.W., 1 alpha, 25-Dihydroxyvitamin D3 inhibits gamma-interferon synthesis by normal human peripheral blood lymphocytes, Proc. Natl. Acad. Sci. U. S. A., 1987, vol. 84, no. 10, pp. 3385–3389.
Rachez, C. and Freedman, L.P., Mechanisms of gene regulation by vitamin D3 receptor: A network of coactivator interactions, Gene, 2000, vol. 246, nos. 1–2, pp. 9–21.
Bhalla, A.K., Amento, E.P., Clemens, T.L., Holick, M.F., and Krane, S.M., Specific high-affinity receptors for 1, 25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation, J. Clin. Endocrinol. Metab., 1983, vol. 57, no. 6, pp. 1308–1310.
Liu, P.T., Schenk, M., Walker, V.P., Dempsey, P.W., Kanchanapoomi, M., Wheelwright, M., et al., Convergence of IL-1β and VDR activation pathways in human TLR2/1-induced antimicrobial responses, PloS One, 2009, vol. 4, no. 6, article no. e5810.
Hansdottir, S., Monick, M.M., Hinde, S.L., Lovan, N., Look, D.C., and Hunninghake, G.W., Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense, J. Immunol., 2008, vol. 181, no. 10, pp. 7090–7099.
Wang, T.-T., Nestel, F.P., Bourdeau, V., Nagai, Y., Wang, Q., Liao, J., et al., Cutting edge: 1, 25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression, J. Immunol., 2004, vol. 173, no. 5, pp. 2909–2912.
Liu, P.T., Stenger, S., Li, H., Wenzel, L., Tan, B.H., Krutzik, S.R., et al., Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response, Science, 2006, vol. 311, no. 5768, pp. 1770–1773.
Park, J.S., The prevalence and risk factors of latent tuberculosis infection among health care workers working in a tertiary hospital in South Korea, Tuberc. Respir. Dis., 2018, vol. 81, no. 4, pp. 274–280.
Keshavarz Valian, S., Mahmoudi, S., Pourakbari, B., Abdolsalehi, M.R., Eshaghi, H., and Mamishi, S., Screening of healthcare workers for latent tuberculosis infection in the low tuberculosis burden country: QuantiFERON-TB gold in tube test or tuberculin skin test?, Arch. Environ. Occup. Health, 2019, vol. 74, no. 3, pp. 109–114.
Vinton, P., Mihrshahi, S., Johnson, P., Jenkin, G.A., Jolley, D., and Biggs, B.A., Comparison of QuantiFERON-TB Gold In-Tube Test and tuberculin skin test for identification of latent Mycobacterium tuberculosis infection in healthcare staff and association between positive test results and known risk factors for infection, Infect. Control Hosp. Epidemiol., 2009, vol. 30, no. 3, pp. 215–221.
Soleimanpour, S., Farsiani, H., Mosavat, A., Ghazvini, K., Eydgahi, M.R.A., Sankian, M., et al., APC targeting enhances immunogenicity of a novel multistage Fc-fusion tuberculosis vaccine in mice, Appl. Microbiol. Biotechnol., 2015, vol. 99, no. 24, pp. 10467–10480.
Funding
This work received specific grant from Mashhad University of Medical Sciences (MUMS).
Author information
Authors and Affiliations
Contributions
Zahra Bagheri and Atieh Yaghoubi contributed equally to this work.
Corresponding authors
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest.
Statement of compliance with standards of research involving humans as subjects. This study was approved by the Research Ethics Committee of Mashhad University of Medical Sciences. Informed consent was obtained from all individual participants involved in the study.
About this article
Cite this article
Bagheri, Z., Yaghoubi, A., Sabet, F. et al. Inconsistency of QuantiFERON-TB Gold Test and Tuberculin Skin Test Results in the Evaluation of Latent Tuberculosis Infection in Health Care Workers. Mol. Genet. Microbiol. Virol. 36, 204–209 (2021). https://doi.org/10.3103/S0891416821040030
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0891416821040030