Abstract
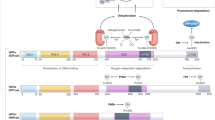
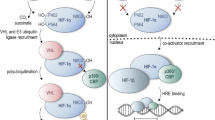
The attempts to elucidate the mechanism of the hypoxic activation of erythropoietin synthesis lead to the discovery of the hypoxia inducible factor (HIF) and its stability regulation via HIF prolyl hydroxylase, iron, and α-ketoglutarate dependent dioxygenase. Enzyme inhibitors mimic the action of hypoxia; however, they are far more specific. Comparative transcriptomic microanalysis of various types of the enzyme inhibitors versus hypoxia at short incubation times demonstrates the potency of inhibitors and points to the primary targets of HIF transcription factor. The power of inhibitors evaluated in the transcriptomic analysis matches the ranking of enzyme inhibitors based on the values of their half-activation constants in the HIF1 ODD-luc reporter assay. Neuradapt is 15–20 times more potent than roxadustat, and in addition, it is much more specific for the target enzyme than roxadustat and dimethyl oxalylglycine, which probably act also on other enzymes of the same family.



Similar content being viewed by others
REFERENCES
Wang, G.L. and Semenza, G.L., Proc. Natl. Acad. Sci. U. S. A., 1993, vol. 90, no. 9, p. 4304. https://doi.org/10.1073/pnas.90.9.4304
Jaakkola, P., Mole, D.R., Tian, Y.-M., Wilson, M.I., Gielbert, J., Gaskell, S.J., and Kriegsheim, A.V., Hebestreit, H.F., Mukherji, M., Schofield, C.J., Maxwell, P.H., Pugh, C.W., and Ratcliffe, P.J., Science, 2001, vol. 292, no. 5516, p. 468. https://doi.org/10.1126/science.1059796
Ivan, M., Kondo, K., Yang, H., Kim, W., Valiando, J., Ohh, M., Salic, A., Asara, J.M., Lane, W.S., and Kaelin, J., Science, 2001, vol. 292, no. 5516, p. 464. https://doi.org/10.1126/science.1059817
Noguchi, C.T., Asavaritikrai, P., Teng, R., and Jia, Y., Crit. Rev. Oncol. Hematol., 2007, vol. 64, no. 2, p. 159. https://doi.org/10.1016/j.critrevonc.2007.03.001
Savyuk, M., Krivonosov, M., Mishchenko, T., Gazaryan, I., Ivanchenko, M., Khristichenko, A., Poloznikov, A., Hushpulian, D., Nikulin, S., Tonevitsky, E., Abuzarova, G., Mitroshina, E., and Vedunova, M., Antioxidants, 2020, vol. 9, no. 8, 662. https://doi.org/10.3390/antiox9080662
Maltseva, D., Raygorodskaya, M., Knyazev, E., Zgoda, V., Tikhonova, O., Zaidi, S., Nikulin, S., Baranova, A., Turchinovich, A., Rodin, S., and Tone-vitsky, A., Biochimie, 2020, vol. 174, p. 107. https://doi.org/10.1016/j.biochi.2020.04.016
Kudriaeva, A., Galatenko, V., Maltseva, D., Khaustova, N., Kuzina, E., Tonevitsky, A., Gabibov, A., and Belogurov, A., Molecules, 2017, vol. 22, no. 5, p. 808. https://doi.org/10.3390/molecules22050808
Chan, M.C., Ilott, N.E., Schodel, J., Sims, D., Tumber, A., Lippl, K., Mole, D.R., Pugh, C.W., Ratcliffe, P.J., Ponting, C.P., and Schofield, C.J., J. Biol. Chem., 2016, vol. 291, no. 39, p. 20661. https://doi.org/10.1074/jbc.M116.749291
Hwang, S., Nguyen, A.D., Jo, Y., Engelking, L.J., Brugarolas, J., and DeBose-Boyd, R.A., J. Biol. Chem., 2017, vol. 292, no. 22, p. 9382. https://doi.org/10.1074/jbc.M117.788562
Kim, H., Kim, D., Choi, S.A., Kim, C.R., Oh, S.K., Pyo, K.E., Kim, J., Lee, S.H., Yoon, J.B., Zhang, Y., and Baek, S.H., Proc. Natl. Acad. Sci. U. S. A., 2018, vol. 115, no. 46, p. 11766. https://doi.org/10.1073/pnas.1805662115
Mellén, M., Ayata, P., and Heintz, N., Proc. Natl. Acad. Sci. U. S. A., 2017, vol. 114, no. 37, E7812. https://doi.org/10.1073/pnas.1708044114
Guo, J., Chen, L., Luo, N., Yang, W., Qu, X., and Cheng, Z., Oncol. Rep., 2015, vol. 33, no. 6, p. 3124. https://doi.org/10.3892/or.2015.3902
Nandadasa, S., Kraft, C.M., Wang, L.W., O’Donnell, A., Patel, R., Gee, H.Y., Grobe, K., Cox, T.C., Hildebrandt, F., and Apte, S.S., Nat. Commun., 2019, vol. 10, p. 953. https://doi.org/10.1038/s41467-019-08520-7
Jan, Y.H., Lai, T.C., Yang, C.J., Huang, M.S., and Hsiao, M., Sci. Rep., 2019, vol. 9, p. 12329. https://doi.org/10.1038/s41598-019-48243-9
Bjorn-Yoshimoto, W.E. and Underhill, S.M., Neurochem. Int., 2016, vol. 98, p. 4. https://doi.org/10.1016/j.neuint.2016.05.007
Qu, Y., Mao, M., Zhao, F., Zhang, L., and Mu, D., Stroke, 2009, vol. 40, no. 8, p. 2843. https://doi.org/10.1161/STROKEAHA.109.553644
Manella, G., Aviram, R., Bolshette, N., Muvkadi, S., Golik, M., Smith, D.F., and Asher, G., Proc. Natl. Acad. Sci. U. S. A., 2020, vol. 117, p. 779. https://doi.org/10.1073/pnas.1914112117
Joshi, A.D., Front. Endocrinol., 2020, vol. 11, p. 172. https://doi.org/10.3389/fendo.2020.00172
Zhang, Y., Shan, P., Srivastava, A., Li, Z., and Lee, P.J., Antioxid. Redox Signaling, 2019, vol. 30, no. 15, p. 1775. https://doi.org/10.1089/ars.2018.7514
Sarapio, E., De Souza, S.K., Model, J.F.A., Trapp, M., and Da, SilvaR.S.M., Can. J. Physiol. Pharmacol., 2019, vol. 97, no. 10, p. 916. https://doi.org/10.1139/cjpp-2019-0023
Benita, Y., Kikuchi, H., Smith, A.D., Zhang, M.Q., Chung, D.C., and Xavier, R.J., Nucleic Acid Res., 2009, vol. 37, no. 14, p. 4587. https://doi.org/10.1093/nar/gkp425
Funding
The study is supported by the Russian Scientific Foundation, grant no. 20-15-00207.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
COMPLIANCE WITH ETHICAL STANDARDS
The article does not describe any research with the participation of animals or humans as subjects of research.
Supplementary Information
There is no supplementary material or additional information.
Additional information
This article was translated by the authors.
About this article
Cite this article
Hushpulian, D.M., Nikulin, S.V., Chubar, T.A. et al. Fast Responding Genes to HIF Prolyl Hydroxylase Inhibitors. Moscow Univ. Chem. Bull. 76, 114–121 (2021). https://doi.org/10.3103/S002713142102005X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S002713142102005X