Abstract
Objective: This study investigated the pharmacokinetics of tretinoin during alternating cycles of 1 week of tretinoin treatment and 1 week drug-free in patients with Ph1+ chronic myelogenous leukaemia (CML) in the chronic phase.
Patients: Eighteen patients with CML were treated with tretinoin 80 mg/m2/day (in two divided doses) for 7 consecutive days every other week (one cycle = 1 week on/1 week off).
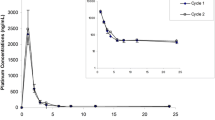
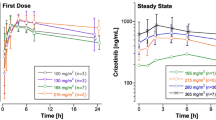
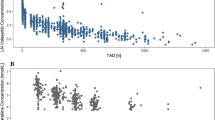
Results: Body systemic exposure to tretinoin as determined by the area under the plasma concentration-time curve (AUC) decreased significantly during the first week of drug administration, from (mean ± SD) 678.3 ± 498.1 to 258.7 ± 272.4 μg/L·h. In about 40% of the patients the decline in plasma concentrations was ≥80%, while 17% of the population did not experience any decline. On day 7 of cycle 1, the mean apparent oral clearance (CL/F) was 2.6 times the corresponding value on day 1. After 1 week without tretinoin, the mean AUC on day 1 of cycle 2 was lower (down 15%) but not statistically different from the corresponding value observed on day 1 of cycle 1; 62% of patients showed an increase in the AUC, which was 40% higher than the corresponding value on day 7 of cycle 1. On day 1 of cycle 6, the AUC and CL/F of tretinoin during a dosage interval were not statistically different from those observed on day 1 of cycle 1 and cycle 2. On all occasions the peak plasma concentration (Cmax) was strongly correlated to the corresponding AUC. No significant change in the time to observed Cmax (tmax) and in the elimination half-life (t½) was observed during the whole study. These results confirmed that the metabolism of tretinoin is rapidly up-regulated in CML patients, with significant declines in plasma drug exposure during the first week of drug administration. After tretinoin was discontinued, a return to the non-induced state followed a mean time-cycle similar to the induction. The strong decrease in the apparent oral drug clearance and the absence of significant variations in the drug half-life demonstrated that the presystemic extraction of tretinoin is the main cause of the marked decline in plasma drug exposure.
Conclusion: The favourable pharmacokinetic profile of tretinoin obtained by an intermittent regimen, 1 week on/1 week off therapy (vs continuous administration), suggests that such a therapeutic schedule is the most appropriate for the assessment of clinical efficacy in those pathologies in which its use is suitable.
Similar content being viewed by others
References
Sporn MB, Roberts AB, Goodman DS. The retinoids: biology, chemistry and medicine. 2nd ed. New York: RavenPressLtd, 1994
Hill DL. Retinoids and cancer prevention. Annu Rev Nutr 1992; 12: 161–81
Sacchi S, Russo D, Avvisati G, et al. All-trans retinoic acid in hematological malignancies, an update. Haematologica 1997; 82(1): 106–21
Warrel RP, Frankel Jr RP, Miller Jr WH, et al. Differentiation therapy of acute promyelocytic leukemia with tretinoin. N Engl J Med 1991; 324(20): 1385–93
Frankel SR, Eardley A, Heller G, et al. All-trans retinoic acid for acute promyelocytic leukemia. Results of the New York study. Ann Intern Med 1994; 120: 278–86
Gillis JC, Goa KL. Tretinoin. A review of its pharmacodynamic and pharmacokinetic properties and use in the management of acute promyelocytic leukemia. Drugs 1995; 50(5): 897–923
Delva L, Cornic M, Balitrand N, et al. Resistance to all-trans retinoic acid (ATRA) therapy in relapsing acute promyelocytic leukemia: study of in vitro ATRA sensitivity and cellular retinoic acid binding protein levels in leukemic cells. Blood 1993; 82: 2175–81
Cornic M, Delva L, Castaigne S, et al. In vitro all-trans retinoic acid sensitivity and cellular retinoic acid binding protein levels in relapse leukemic cells after remission induction by ATRA in acute promyelocytic leukemia. Leukemia 1994; 8: 914–7
Warrell RP. Retinoid resistance in acute promyelocytic leukemia: new mechanisms, strategies and implications. Blood 1993; 82(7): 1949–53
Muindi JRF, Young CW, Warrell RP. Clinical pharmacology of all-trans-retinoic acid. Leukemia 1994; 8: 1807–12
Adamson PC. Pharmacokinetics of all-trans retinoic acid: clinical implications in acute promyelocytic leukemia, Semin Hematol 1994; 31(5): 14–7
Adamson PC. Clinical and pharmacokinetic studies of all-trans retinoic acid in pediatric patients with cancer. Leukemia 1994; 8(11): 1813–6
Regazzi BM, Iacona I, Gervasutti C, et al. Clinical pharmacokinetics of tretinoin. Clin Pharmacokinet 1997; 32(5): 382–402
Trump DL, Smith DC, Stiff D, et al. A phase II trial of all-trans retinoic acid in hormone refractory prostate cancer: a clinical trial with detailed pharmacokinetic analysis. Cancer Chemother Pharmacol 1997; 39(4): 349–56
Muindi J, Frankel SR, Miller WH, et al. Continuous treatment with all-trans retinoic acid results in a progressive decrease in plasma concentrations: implications for relapse and retinoid ‘resistance’ in acute promyelocytic leukemia. Blood 1992; 79: 299–303
Agadir A, Cornic M, Lefebvre P, et al. All-trans retinoic acid pharmacokinetics and bioavailability in acute promyelocytic leukemia: intracellular concentrations and biological response relationship. J Clin Oncol 1995; 13(10): 2517–23
Adamson PC, Boylan JF, Balis FM, et al. Time course of induction of metabolism of all-trans-retinoic acid and the up-regulation of cellular retinoic acid-binding protein Cancer Res 1993; 53: 472–6
Cornic M, Delva L, Guidez F, et al. Induction of retinoic acid- binding protein in normal and malignant human myeloid cells by retinoic acid in acute promyelocytic leukemia patients. Cancer Res 1992; 52: 3329–34
Wadler S, Schwartz EL, Haynes H, et al. All-trans retinoic acid and interferon-ot-2a in patients with metastatic or recurrent carcinoma of the uterine cervix. Cancer 1997; 79(8): 1574–80
Sutton LM, Warmuth MA, Petros WP, et al. Pharmacokinetics and clinical impact of all-trans retinoic acid in metastatic breast cancer: a phase II trial. Cancer Chemother Pharmacol 1997; 40: 335–41
Adamson PC, Bailey J, Pluda J, et al. Pharmacokinetics of all-trans-retinoic acid administered on an intermittent schedule. J Clin Oncol 1995; 13: 1238–40
Lee JS, Newman RA, Lippman SM, et al. Phase I evaluation of all-trans retinoic acid with and without ketokonazole in adults with solid tumors. J Clin Oncol 1995; 13(6) 1501–8
Toma S, Iacona I, Palumbo R, et al. Modulation of all-trans retinoic acid administered on intermittent schedule by α-interferon 2a in a patient with AIDS-related Kaposi's sarcoma. AIDS 1996; 10(9): 1049–50
Kizaki M, Ueno H, Yamazoe Y, et al. Mechanisms of retinoid resistance in leukemic cells: possible role of cytochrome P450 and P-glycoprotein. Blood 1996; 87(2): 725–33
Mehta K, Sadeghi T, McQueen T, et al. Liposome encapsulation circumvents the hepatic clearance mechanisms of all-trans retinoic acid. Leukemia Res 1994; 18(8): 587–96
Barua AB, Barres RO, Olson JA. Characterization of retinyl β-glucuronide in human blood. Am J Clin Nutr 1989; 50: 370–4
Sacchi S, Regazzi MB, Awisati G, et al. Phase I/II study of the biological activity of all-trans retinoic acid in patients with Ph1 + chronic myeloid leukemia [abstract 3532]. Blood 1996; 88(10 Suppl. 1): 201b
Adamson PC, Pitot HC, Balis F, et al. Variability in the oral bioavailability of all-trans retinoic acid. J Natl Cancer Inst 1993; 85: 993–6
Fiorella PD, Napoli JL. Microsomal retinoic acid metabolism. J Biol Chem 1994; 269: 10538–44
Data on file, F. Hoffmann-La Roche, Basel, Switzerland
Rowland M, Tozer T. Clinical pharmacokinetics, concepts and applications. 1st ed. Philadelphia, Lea &Febiger, 1980: 138–50
Cullum ME, Zile MH. Metabolism of all-trans retinoic acid and all-trans retinyl acetate. J Biol Chem 1985; 260: 305–12
Davies DS, Ilett KF, George CF. Drug metabolism by intestinal mucosa. In: Prescott LF, Nimmo WS, editors. Drug absorption. The Proceedings of the International Conference on Drug Absorption, Edinburgh, September 1979. Balgowlah, Australia: ADIS Press, 1979: 73–9
el Mansouri S, Tod M, Leclerq M, et al. Time and dose-dependent kinetics of all-trans-retinoic acid in rats after oral or intravenous administration(s). Drug Metab Dispos 1995; 23(2): 227–31
Roberts AB, Nichols MD, Newton DL, et al. In vitro metabolism of retinoic acid in hamster intestine and liver. J Biol Chem 1979; 254: 6296–302
Thiebaut F, Tsuruo T, Hamada H, et al. Cellular localization of the multidrug resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A 1987; 84: 7735–8
Benet LZ. Efflux proteins and drug metabolism. Eur J Pharmaceut Sci 1997; 5Suppl. 2: S11
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Regazzi, M.B., Russo, D., Iacona, I. et al. Time-Dependent Kinetics of Tretinoin in Chronic Myelogenous Leukaemia during Intermittent Dose Scheduling. Clin. Drug Investig. 16, 25–33 (1998). https://doi.org/10.2165/00044011-199816010-00004
Published:
Issue Date:
DOI: https://doi.org/10.2165/00044011-199816010-00004