Abstract
Setting
In Canada’s federated healthcare system, 13 provincial and territorial jurisdictions have independent responsibility to collect data to inform health policies. During the COVID-19 pandemic (2020–2023), national and regional sero-surveys mostly drew upon existing infrastructure to quickly test specimens and collect data but required cross-jurisdiction coordination and communication.
Intervention
There were 4 national and 7 regional general population SARS-CoV-2 sero-surveys. Survey methodologies varied by participant selection approaches, assay choices, and reporting structures. We analyzed Canadian pandemic sero-surveillance initiatives to identify key learnings to inform future pandemic planning.
Outcomes
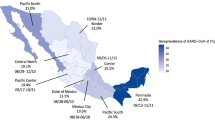
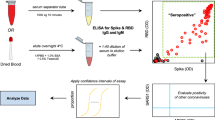
Over a million samples were tested for SARS-CoV-2 antibodies from 2020 to 2023 but siloed in 11 distinct datasets. Most national sero-surveys had insufficient sample size to estimate regional prevalence; differences in methodology hampered cross-regional comparisons of regional sero-surveys. Only four sero-surveys included questionnaires. Sero-surveys were not directly comparable due to different assays, sampling methodologies, and time-frames. Linkage to health records occurred in three provinces only. Dried blood spots permitted sample collection in remote populations and during stay-at-home orders.
Implications
To provide timely, high-quality information for public health decision-making, routine sero-surveillance systems must be adaptable, flexible, and scalable. National capability planning should include consortiums for assay design and validation, defined mechanisms to improve test capacity, base documents for data linkage and material transfer across jurisdictions, and mechanisms for real-time communication of data. Lessons learned will inform incorporation of a robust sero-survey program into routine surveillance with strategic sampling and capacity to adapt and scale rapidly as a part of a comprehensive national pandemic response plan.
Résumé
Contexte
Au Canada, où le système de santé est fédéré, les 13 juridictions provinciales et territoriales ont la responsabilité individuelle de recueillir les données qui leur permettent d’élaborer leurs politiques de santé. Lors de la pandémie de COVID-19 (2020–2023), pour réaliser les enquêtes de séroprévalence à l’échelle régionale et nationale, les autorités ont principalement utilisé l’infrastructure existante pour pouvoir analyser les échantillons et recueillir des données rapidement, mais cela a également nécessité de la communication et de la coordination entre les différentes juridictions.
Intervention
Au Canada, il y a eu quatre enquêtes nationales et sept enquêtes régionales sur la séroprévalence du SARS-CoV-2 dans la population générale. Les méthodologies utilisées différaient selon la méthode de sélection des participants, le choix des tests d’analyses et les structures de rapports. Nous avons analysé la façon dont ces enquêtes avaient été réalisées afin d’en dégager des éléments essentiels qui permettront de planifier pour les futures pandémies.
Résultats
Entre 2020 et 2023, plus d’un million d’échantillons, répartis en 11 ensembles de données distincts, ont été analysés afin de rechercher la présence d’anticorps au SARS-CoV-2. Dans la plupart des enquêtes nationales, la taille de l’échantillon était insuffisante pour pouvoir estimer la prévalence à l’échelle régionale. La disparité des méthodologies utilisées a entravé la comparaison des enquêtes régionales. Seules quatre enquêtes fournissaient les données recueillies à partir des questionnaires. Il a été impossible de comparer les enquêtes entre elles en raison de la diversité des tests d’analyse utilisés, des méthodes d’échantillonnage et de la durée des enquêtes. Seules trois provinces avaient couplé leurs données avec les archives médicales. Pour réaliser les enquêtes dans les populations éloignées et lors des périodes de confinement, la méthode d’analyse sur gouttes de sang séché a été utilisée.
Conclusion
Afin de pouvoir fournir, en temps et en heure, des données de haute qualité pour la prise de décisions en matière de santé publique, un système de sérosurveillance continuelle doit être adaptable, modulable et évolutif. En cas de pandémie, un plan national doit prévoir des consortiums pour la conception et la validation des tests d’analyse, des moyens d’amélioration de la capacité de dépistage, des documents de base pour le couplage des données, un mode de transfert du matériel entre les différentes juridictions et des moyens pour une communication en temps réel des données. Les leçons tirées de cette analyse permettront de mettre en place un solide programme d’enquêtes de séroprévalence au sein des systèmes de sérosurveillance continuelle, et que ce programme sera accompagné d’une stratégie d’échantillonnage et d’un plan d’intervention national, rapide et complet en cas de pandémie.

Similar content being viewed by others
Data availability
N/A.
Code availability
N/A.
References
Allin, S., Fitzpatrick, T., Marchildon, G. P., & Quesnel-Vallée, A. (2022). The federal government and Canada’s COVID-19 responses: From ‘we’re ready, we’re prepared’ to ‘fires are burning.’ Health Economics, Policy, and Law, 17(1), 76–94.
Atkinson, A., Albert, A., McClymont, E., Andrade, J., Beach, L., Bolotin, S., et al. (2023). Canadian SARS-CoV-2 serologic survey using antenatal serum samples: A retrospective seroprevalence study. CMAJ Open, 11(2), E305–E313.
Blauer, B., Brownstein, J. S., Gardner, L., Kraemer, M. U. G., Mathieu, E., Redies, I., et al. (2023). Innovative platforms for data aggregation, linkage and analysis in the context of pandemic and epidemic intelligence. Eurosurveillance Weekly, 28, 2200860.
Bobrovitz, N., Ware, H., Ma, X., Li, Z., Hosseini, R., Cao, C., et al. (2023). Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the omicron variant and severe disease: A systematic review and meta-regression. The Lancet Infectious Diseases, 23(5), 556–567.
Bushnik, T., Earl, S., Cabot, J., & Clarke, J. (2022). COVID-19 infection in the Canadian household population. Health Reports, 33(4), 24–33.
Charlton, C. L., Nguyen, L. T., Bailey, A., Fenton, J., Plitt, S. S., Marohn, C., et al. (2021). Pre-vaccine positivity of SARS-CoV-2 antibodies in Alberta, Canada during the first two waves of the COVID-19 pandemic. Microbiology Spectrum, 9, e00291-e321.
Cholette, F., Mesa, C., Harris, A., Ellis, H., Cachero, K., Lacap, P., et al. (2021). Dried blood spot specimens for SARS-CoV-2 antibody testing: A multi-site, multi-assay comparison. PLoS ONE, 16(12), e0261003.
Cholette, F., Fabia, R., Harris, A., Ellis, H., Cachero, K., Schroeder, L., et al. (2022). Comparative performance data for multiplex SARS-CoV-2 serologic assays from a large panel of dried blood spot specimens. Heliyon, 8(9), e10270.
CITF. (2020). COVID-19 Immunity Task Force. Available at https://www.covid19immunitytaskforce.ca/. Accessed 10 Jun 2024.
Dibernardo, A., Toledo, N. P., Robinson, A., Osiowy, C., Giles, E., Day, J., et al. (2022). Evaluation of the performance of multiple immunoassay diagnostic platforms on the National Microbiology Laboratory SARS-CoV-2 National Serology Panel. Journal of the Association of Medical Microbiology and Infectious Disease Canada, 7(3), 186–195.
Duong, S., Burtniak, J., Gretchen, A., Mai, A., Klassen, P., Wei, Y., et al. (2023). Riding high: Seroprevalence of SARS-CoV-2 after 4 pandemic waves in Manitoba, Canada, April 2020-February 2022. BMC Public Health, 23, 2420.
Expert Advisory Group to the Government of Canada. (2022). The pan-Canadian Health Data Strategy: Toward a world-class health data system. Available at: https://www.canada.ca/en/public-health/corporate/mandate/about-agency/external-advisory-bodies/list/pan-canadian-health-data-strategy-reports-summaries.html. Accessed 10 Jan 2024.
Frei, A., Kaufmann, M., Amati, R., Butty Dettwiler, A., von Wyl, V., Annoni, A. M., et al. (2023). Development of hybrid immunity during a period of high incidence of Omicron infections. International Journal of Epidemiology, 52(6), 1696–1707.
Government of Canada. (1985). Canada Health Act. Available at https://www.canada.ca/en/health-canada/services/health-care-system/canada-health-care-system-medicare/canada-health-act.html. Accessed 10 Jan 2024.
Hasing, M. E., Lee, B. E., Gao, T., Li, Q., Qiu, Y., Ellehoj, E., et al. (2023). Wastewater surveillance monitoring of SARS-CoV-2 variants of concern and dynamics of transmission and community burden of COVID-19. Emerging Microbes & Infections, 12, 2233638.
Isho, B., Abe, K. T., Zuo, M., Jamal, A. J., Rathod, B., Wang, J. H., et al. (2020). Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Science Immunology, 5(52), eabe5511.
Kalish, H., Klumpp-Thomas, C., Hunsberger, S., Baus, H. A., Fay, M. P., Siripong, N., et al. (2021). Undiagnosed SARS-CoV-2 seropositivity during the first 6 months of the COVID-19 pandemic in the United States. Science Translational Medicine, 13, eabh3826.
Kanji, J. N., Nguyen, L. T., Plitt, S. S., Charlton, C. L., Fenton, J., Braun, S., et al. (2022). Seropositivity to SARS-CoV-2 in Alberta, Canada in a post-vaccination period (March 2021 – July 2021). Infectious Diseases (lond), 54(9), 666–676.
Lewin, A., Therrien, R., De Serres, G., Gregoire, Y., Perreault, J., Drouin, M., et al. (2021). SARS-CoV-2 seroprevalence among blood donors in Québec, and analysis of symptoms associated with seropositivity: A nested case-control study. Canadian Journal of Public Health, 112(4), 576–586.
McRae, A. D., Archambault, P., Fok, P., Wiemer, H., Morrison, L. J., Herder, M., et al. (2022). Reducing barriers to accessing administrative data on SARS-CoV-2 vaccination for research. CMAJ, 194(27), E943-947.
Murphy, T., Swail, H., Jain, J., Anderson, M., Awadalla, P., Behl, L., et al. (2023). The evolution of SARS-CoV-2 seroprevalence in Canada: A time-series study, 2020–2023. CMAJ, 195(31), E1030-E1037.
National Advisory Committee on Immunization. (2023). Available at: https://www.canada.ca/en/public-health/services/immunization/national-advisory-committee-on-immunization-naci.html. Accessed 10 Jan 2024.
O’Brien, S. F., Caffrey, N., Yi, Q. L., Pambrun, C., & Drews, S. J. (2022a). SARS-CoV-2 seroprevalence among Canadian blood donors: The advance of Omicron. Viruses, 14(11), 2336.
O’Brien, S. F., Drews, S. J., Lewin, A., Osiowy, C., Drebot, M. A., & Renaud, C. (2022b). Canadian blood suppliers: An expanding role in public health surveillance? Canada Communicable Disease Report, 48(4), 124–130.
Perreault, J., Tremblay, T., Fournier, M. J., Drouin, M., Beaudoin-Bussieres, G., Prevost, J., Lewin, A., Begin, P., Finzi, A., & Bazin, R. (2020). Waning of SARS-CoV-2 RBD antibodies within four months after symptom onset. Blood, 136(22), 2588–2591.
Sero-tracker. (2020). Available at: https://serotracker.com/en/Explore. Accessed 10 Jan 2024.
Skowronski, D. M., Kaweski, S. E., Irvine, M. A., Kim, S., Chuang, E. S. Y., Sabaiduc, S., et al. (2022). Serial cross-sectional estimation of vaccine-and infection-induced SARS-CoV-2 seroprevalence in British Columbia, Canada. CMAJ, 194(47), E1599–E1609.
Tang, X., Sharma, A., Pasic, M., Brown, P., Colwill, K., Gelband, H., et al. (2022). Assessment of SARS-CoV-2 seropositivity during the first and second viral waves in 2020 and 2021 among Canadian adults. JAMA Network Open, 5(2), e2146798.
Urquhart, R., Awadalla, P., Bhatti, P., Drummer, T., Gravel, S., Vena, J., et al. (2022). Harnessing the power of data linkage to enrich the cancer research ecosystem in Canada. International Journal of Population Data Science, 7(3), 176.
Wu, S. L., Mertens, A. N., Crider, Y. S., Nguyen, A., Pokpongkiat, N. N., Djajadi, S., et al. (2020). Substantial underestimation of SARS-CoV-2 infection in the United States. Nature Communications, 11(1), 4507.
**a, Y., Ma, H., Moloney, G., Valasquex Garcia, H. A., Sirski, M., Janjua, N. Z., et al. (2022). Geographic concentration of SARS-CoV-2 cases by social determinants of health in metropolitan areas in Canada: A cross-sectional study. CMAJ, 194(6), E195-E204.
Author information
Authors and Affiliations
Contributions
All authors conceived the commentary. SFO and MAB reviewed Canadian sero-surveys and prepared the tables and graph. All authors contributed to analysis and interpretation of the sero-surveys. All authors contributed to development of recommendations for future pandemic preparedness. SFO and MAB prepared the first draft of the manuscript. All authors critically revised and approved the final draft.
Corresponding author
Ethics declarations
Ethics approval
N/A.
Consent to participate
N/A.
Consent for publication
N/A.
Conflict of interest
Drews has received laboratory supplies from Abbott Pharmaceuticals and consulting fees from Roche.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
O’Brien, S.F., Asamoah-Boaheng, M., Grunau, B. et al. Canada’s approach to SARS-CoV-2 sero-surveillance: Lessons learned for routine surveillance and future pandemics. Can J Public Health (2024). https://doi.org/10.17269/s41997-024-00901-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.17269/s41997-024-00901-w