Abstract
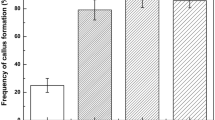
A tissue culture and plant regeneration protocol for the salt marsh grass, Spartina alterniflora, has been developed. Callus was efficiently induced on Murashige and Skoog (MS) medium supplemented with 1 mg L−1 2,4-dichlorophenoxyacetic acid (2,4-D) and 1 mg L−1 indole-3-acetic acid (IAA). Callus initiation from 6-day-old seedlings was faster and occurred with a greater frequency than callus initiation from coleoptile-covered segments from the same age seedlings. However, only the coleoptile-covered segments produced regenerable callus, which was maintained on MS medium supplemented with 1 mg L−1 2,4-D and 1 mg L−1 naphthaleneacetic acid (NAA). The regenerable callus differentiated into shoots upon transfer to shoot regeneration medium. A high frequency of shoot regeneration was obtained when the medium contained 3 mg L−1 6-benzylaminopurine (BA) or Thidiazuron (TDZ), with or without the addition of 0.2 mg L−1 IAA. Regenerated shoots were transferred to root regeneration medium, the optimal of which was determined to be half-strength MS medium supplemented with 1 mg L−1 indole-3-butyric acid (IBA). TDZ in the shoot regeneration medium inhibited root formation in the root regeneration medium, making BA rather than TDZ the optimal hormone for the shoot regeneration medium. The mode of plant regeneration was organogenesis. Upon transfer to soil, the most successful growth of plants occurred in a mixture of commercially available potting soil and natural marsh mud. The development of a tissue culture and regeneration protocol for S. alterniflora provides the possibility of selecting lines of this species, via somaclonal variation, with characteristics desirable for wetland creation and restoration.
Similar content being viewed by others
Literature Cited
Adkins, S. W., R. Kunanuvatchaidach, and I. D. Godwin. 1995. Somaclonal variation in rice drought tolerance and other agronomic characters. Australian Journal of Botany 43:201–209.
Arnold, D., A. Flegmann, and J. M. Clarkson. 1995. Somaclonal variation in watercress for resistance to crook root disease. Plant Cell Reports 14:241–244.
Bertin, P., J. Bouharmont, and J. M. Kinet. 1997. Somaclonal variation and improvement of chilling tolerance in rice: Changes in chilling-induced chlorophyll fluorescence. Crop Science 37:1727–1735.
Chambers, R. M., T. J. Mozdzer, and J. C. Ambrose. 1998. Effects of salinity and sulfide on the distribution of Phragmites australis and Spartina alterniflora in a tidal salt marsh. Aquatic Botany 62:161–169.
Chintapalli, P. L., J. P. Moss, K. K. Sharma, and J. K. Bhalla. 1997. In vitro culture provides additional variation for pigeonpea (Cajanus cajan L.) Millsp.) crop improvement. In Vitro Cellular and Developmental Biology-Plant 33:30–37.
Cook, D. A., D. M. Decker, and J. L. Gallagher. 1989. Regeneration of Kosteletzkya virginica (L.) Presl. Seashore Mallow from callus cultures. Plant Cell Tissue and Organ Culture 17:111–119.
Freshwater, D. W. 1988. Relative genome-size differences among populations of Spartina alterniflora Loisel. (Poaceae) along East and Gulf Coasts of USA. Journal of Experimental Marine Biology and Ecology 120:239–246.
Gallagher, J. L. and F. G. Plumley. 1979. Underground biomass profiles and productivity in Atlantic Coastal Marshes. American Journal of Botany 66:156–161.
Gallagher, J. L., R. J. Reimold, R. A. Linthurst, and W. J. Pfeiffer. 1980. Acrial production, mortality and mineral accumulation dynamics in Spartina alterniflora and Juncus roemerianus in a Georgia salt marsh. Ecology 61:303–312.
Gallagher, J. L., G. F. Somers, D. M. Grant, and D. M. Seliskar. 1988. Persistent differences in Spartina alterniflora growth forms. Ecology 69:1005–1008.
Larkin, P. J. and W. R. Scowcroft. 1981. Somaclonal variation-a noval source of variability from cell culture for plant improvement. Theoretical and Applied Genetics 60:1967–1214.
Li, X. and J. L. Gallagher. 1996a. Expression of foreign genes, GUS and hygromycin resistance, in the halophyte Kosteletzkya virginiea in response to bombardment with the Particle Inflow Gun. Journal of Experimental Botany 47:1437–1447.
Li, X. and J. L. Gallagher. 1996b. Tissue culture and plant regeneration of big cordgrass, Spartina cynosuroides: implications for wetland restoration. Wetlands 16:410–415.
Li, X., D. M. Seliskar, J. Moga, and J. L. Gallagher. 1995. Plant regeneration from callus cultures of salt marsh hay, Spartina patens, and its cellular-based salt tolerance. Aquatic Botany 51:103–113.
McClintock, B. 1984. The significance of responses of the genome to challenge. Science 226:792–801.
Mendelssohn, I. A., K. L. McKee, and W. H. Patrick. 1981. Oxygen deficiency in Spartina alterniflora roots: metabolic adaptation to anoxia. Science 214:439–441.
Mooring, M. T., A. W. Cooper, and E. D. Seneca. 1971. Seed germination response and evidence for height ecophenes in Spartina alterniflora from North Carolina. American Journal of Botany 58:48–55.
Murashige, T. and F. Skoog. 1962. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiologia Plantarum 15:473–497.
Nestler, J. 1977. Interstitial salinity as a cause of ecphenic variation in Spartina alterniflora. Estuarine and Coastal Marine Science 5:46–55.
Oberthur, L. E., S. A. Harrison, T. P. Croughan, and D. L. Long. 1993. Inheritance of improved leaf rust resistance in somaclones of wheat. Crop Science 33:444–448.
O’Brien, D. L and D. M. Freshwater. 1999. Genetic diversity within tall form Spartina alterniflora Loisel. along the Atlantic and Gulf coasts of the United States. Wetlands 19:352–358.
Pezeshki, S. R. 1995. Variations in response of two U.S. gulf coast populations of Spartina alterniflora to hypersalinity. Journal of Coastal Research 11:89–95.
Racchi, M. L., M. Rebecchi, G. Todesco, E. Nielsen, and G. Forlani. 1995. Glyphosate tolerance in maize (Zea mays L.): 2. Selection and characterization of a tolerant somaclone. Euphytica 82:165–173.
Rogers, S. M. D., J. Beech, and K. S. Sarma. 1998. Shoot regeneration and plant acclimatization of the wetland monocot Cattail (Typha latifolia). Plant Cell Reports 18:71–75.
Roylance, J. T., N. S. Hill, and W. A. Parrott. 1994. Detection of somaclonal variation in tissue-culture regenerants of tall fescue. Crop Science 34:1369–1372.
Saltman, T. and J. L. Gallagher. 1998. An analysis of Phragmites use in municipal sludge drying beds. American Journal of Botany (Abstract) 85:40.
Sarma, K. S. and S. M. D. Rogers. 1998. Plant regeneration and multiplication of the emergent wetland monocot Juncus accuminatus. Plant Cell Reports 17:656–660.
Schubauer, J. P. and C. S. Hopkinson. 1984. Above- and belowground emergent macrophyte production and turnover in a coastal marsh ecosystem, Georgia. Limnology and Oceanography 29:1052–1065.
Seliskar, D. M. 1995. Exploiting plant genotypic diversity for coastal saltmarsh creation and restoration. p. 407–416. In Biology of Salt-Tolerant Plants. M.A. Khan and I.A. Ungar (eds.) Department of Botany, University of Karachi, Karachi, Pakistan.
Seliskar, D. M. 1998. Natural and tissue culture-generated variation in the salt marsh grass Sporobolus virginicus: Potential selections for marsh creation and restoration. HortScience 33:622–625.
Seliskar, D. M. and J. L. Gallagher. 2000. Exploiting wild population diversity and somaclonal variation in the salt marsh grass Distichlis spicata (Poaceae) for marsh creation and restoration. American Journal of Botany 87:141–146.
Seliskar, D. M., J. L. Gallagher, D. M. Burdick, and L. M. Mutz. 2002. The regulation of ecosystem functions by ecotypic variation in the dominant plant: A Spartina alterniflora salt marsh case study. Journal of Ecology 90:1–11.
Seneca, E. D. 1974. Germination and seedling response of Atlantic and Gulf coasts populations of Spartina alterniflora. American Journal of Botany 61:947–956.
Shan, X., D. Li, and R. Qu. 2000. Thidiazuron promotes in vitro regeneration of wheat and barley. In vitro Cellular and Developmental Biology-Plant 36:207–210.
Somers, G. F. and D. Grant. 1981. Influence of seed source upon phenology of flowering of Spartina alterniflora Loisel. and the likelihood of cross pollination. American Journal of Botany 68:6–9.
Straub, P. F., J. L. Gallagher, and D. M. Decker. 1988. Tissue culture and long-term regeneration of Phragmites australis (Cav.) Trin. ex Steud. Plant Cell Tissue and Organ Culture 15:73–78.
Straub, P. F., D. M. Decker, and J. L. Gallagher. 1989. Tissue culture and regeneration of Distichlis spicata (Gramineae). American Journal of Botany 76:1448–1451.
Straub, P. F., D. M. Decker, and J. L. Gallagher. 1992. Characterization of tissue culture initiation and plant regeneration in Sporobolus virginicus (Gramineae). American Journal of Botany 79:1119–1125.
Wijte, A. H. B. M. and J. L. Gallagher. 1996a. Effect of oxygen availability and salinity on early life history stages of salt marsh plants. I: Different germination strategies of Spartina alterniflora and Phragmites australia (Poaceae). American Journal of Botany 83:1337–1342.
Wijte, A. H. B. M. and J. L. Gallagher. 1996b. Effect of oxygen availability and salinity on early life history stages of salt march plants. II. Early seedling development advantage of Spartina alterniflora over Phragmites australis (Poaceae). American Journal of Botany 83:1343–1350.
Winicov, I. 1991. Characterization of salt tolerant alfalfa (Medicago Sativa L.) plants regenerated from salt tolerant cell lines. Plant Cell Reports 10:561–564.
Woodhouse, W. W. 1979. Building salt marshers along the coasts of the continental United States. U. S. Army Corps of Engineers, Coastal Engineering Research Center, Belvoir, VA. Special report.
Wu, J. and D. M. Seliskar. 1998. Salinity adaptation of plasma membrane H+-ATPase in the salt marsh plant Spartina patens: ATP hydrolysis and enzyme kinetics. Journal of Experimental Botany 49:1005–1013.
Wu, J., D. M. Seliskar, and J. L. Gallagher. 1998. Stress tolerance in the marsh plant Spartina patens: Impact of NaCl on growth and root plasma membrane lipid composition. Physiologia Plantarum 102:307–317.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wang, J., Seliskar, D.M. & Gallagher, J.L. Tissue culture and plant regeneration of Spartina alterniflora: Implications for wetland restoration. Wetlands 23, 386–393 (2003). https://doi.org/10.1672/0277-5212(2003)023[0386:TCAPRO]2.0.CO;2
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1672/0277-5212(2003)023[0386:TCAPRO]2.0.CO;2