Abstract
Objective
Labisia pumila var. alata, commonly known as ‘Kacip Fatimah’ or ‘Selusuh Fatimah’ in Southeast Asia, is traditionally used by members of the Malay community because of its post-partum medicinal properties. Its various pharmaceutical applications cause an excessive harvesting and lead to serious shortage in natural habitat. Thus, this in vitro propagation study investigated the effects of different plant growth regulators (PGRs) on in vitro leaf and stem explants of L. pumila.
Methods
The capabilities of callus, shoot, and root formation were evaluated by culturing both explants on Murashige and Skoog (MS) medium supplemented with various PGRs at the concentrations of 0, 1, 3, 5, and 7 mg/L.
Results
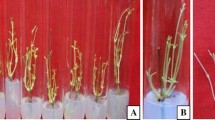
Medium supplemented with 3 mg/L indole-3-butyric acid (IBA) showed the optimal callogenesis from both leaf and stem explants with (72.34±19.55)% and (70.40±14.14)% efficacy, respectively. IBA was also found to be the most efficient PGR for root induction. A total of (50.00±7.07)% and (77.78±16.47)% of root formation were obtained from the in vitro stem and leaf explants after being cultured for (26.5±5.0) and (30.0±8.5) d in the medium supplemented with 1 and 3 mg/L of IBA, respectively. Shoot formation was only observed in stem explant, with the maximum percentage of formation ((100.00±0.00)%) that was obtained in 1 mg/L zeatin after (11.0±2.8) d of culture.
Conclusions
Callus, roots, and shoots can be induced from in vitro leaf and stem explants of L. pumila through the manipulation of types and concentrations of PGRs.
Similar content being viewed by others
References
Abdul Kadir, A., Nik Hussain, N.H., Wan Bebakar, W.M., Mohd, D.M., Wan Mohammad, W.M.Z., Hassan, I.I., Shukor, N., Kamaruddin, N.A., Wan Mohd, W.N., 2012. The effect of Labisia pumila var. alata on postmenopausal women: a pilot study. Evid. Based Complement. Alternat. Med., 2012:216525. [doi:10.1155/2012/216525]
Aggarwal, D., Kumar, A., Reddy, M.S., 2010. Shoot organogenesis in elite clones of Eucalyptus tereticornis. Plant Cell Tiss. Org. Cult., 102(1):45–52. [doi:10.1007/s11240-010-9703-y]
Auer, C.A., Motyka, V., Březinová, A., Kamínek, M., 1999. Endogenous cytokinin accumulation and cytokinin oxidase activity during shoot organogenesis of Petunia hybrida. Physiol. Plant., 105(1):141–147. [doi:10.1034/j.1399-3054.1999.105121.x]
Christensen, B., Sriskandarajah, S., 2008. In vitro culture of Hibiscus rosa-sinensis L.: influence of iron, calcium and BAP on establishment and multiplication. Plant Cell Tiss. Org. Cult., 93(2):151–161. [doi:10.1007/s11240-008-9354-4]
Conde, P., Sousa, A., Costa, A., Santos, C., 2008. A protocol for Ulmus minor Mill. micropropagation and acclimatization. Plant Cell Tiss. Org. Cult., 92(1):113–119. [doi:10. 1007/s11240-007-9310-8]
Cozza, R., Turco, D., Bati, C.B., Bitonti, M.B., 1997. Influence of growth medium on mineral composition and leaf histology in micropropagated plantlets of Olea europaea. Plant Cell Tiss. Org. Cult., 51(3):215–223. [doi:10.1023/A:1005966404642]
Cunha, A.C.G.D., Ferreira, M.F., 1996. Somatic embryogenesis, organogenesis and callus growth kinetics of flax (Linum usitatissium L.). Plant Cell Tiss. Org. Cult., 47(1):1–8. [doi:10.1007/BF02318959]
Debnath, S.C., 2008. Zeatin-induced one-step in vitro cloning affects the vegetative growth of cranberry (Vaccinium macrocarpon Ait.) micropropagules over stem cuttings. Plant Cell Tiss. Org. Cult., 93(2):231–240. [doi:10. 1007/s11240-008-9366-0]
Debnath, S.C., McRae, K.B., 2005. A one-step in vitro cloning procedure for cranberry (Vaccinium macrocarpon Ait.): the influence of cytokinins on shoot proliferation and rooting. Small Fruits Rev., 4(3):57–75. [doi:10.1300/J301v04n03_05]
Dieleman, J.A., Verstappen, F.W.A., Nicander, B., Kuiper, D., Tillberg, E., Tromp, J., 1997. Cytokinins in Rosa hybrida in relation to bud break. Physiol. Plant., 99(3):456–464. [doi:10.1111/j.1399-3054.1997.tb00560.x]
Eccher, T., Noe, N., 1989. Comparison between 2iP and zeatin in the micropropagation of highbush blueberry (Vaccinium corymbosum). Acta Hort., 241:185–190.
Evans, D.E., Coleman, J.O.D., Kearns, A., 2003. Callus Cultures. In: Basics Plant Tissue Culture. BIOS Scientific Publishers, New York, p.63–67.
Farouk, A.E., Nawi, M.N., Hassan, S., 2008. Antibacterial peptides from Euycoma longifolia (Tongkat Ali) and Labisia pumila (Kacip Fatimah) leaves in Malaysia. Sci. Brun., 9:55–63.
Husen, A., Pal, M., 2007. Metabolic changes during adventitious root primordium development in Tectona grandis Linn. f. (teak) cuttings as affected by age of donor plants and auxins (IBA and NAA) treatment. New Forests, 33(3):309–323. [doi:10.1007/s11056-006-9030-7]
Imin, N., Nizamidin, M., Daniher, D., Nolan, K.E., Rose, R.J., Rolfe, B.G., 2005. Proteomic analysis of somatic embryogenesis in Medicago truncatula explant cultures grown under 6-benzylaminopurine and 1-naphthaleneacetic acid treatments. Plant Physiol., 137(4):1250–1260. [doi:10. 1104/pp.104.055277]
Jamia, A.J., Houghton, P.J., Milligan, S.R., Ibrahim, J., 2003. The oestrogenic and cytotoxic effects of the extracts of Labisia pumila and Labisia pumila var. pumila in vitro. J. Sains Kesihatan Malaysia, 1:53–60.
JIRCAS-SFD Joint Research Project, 2007. Agroforestry Approach to the Rehabilitation of Tropical Lands by Using Nurse Trees. JIRCAS-Sabah Forestry Department (SFD), Malaysia.
Khalafalla, M.M., Gaali, E.E.I., Abbas, F.M., Ali, H.A., 2007. Neem (Azadirachta indica A. Juss) callus induction and its larvaecidal activity against Anopheles mosquito. Int. J. Biotechnol. Biochem., 3(1):85–94.
Khalafalla, M.M., Daffalla, H.M., El-Shemy, H.A., Abdellatef, E., 2009. Establishment of in vitro fast-growing normal root culture of Vernonia amygdalina-a potent African medicinal plant. Afr. J. Biotechnol., 8(2):5952–5957.
Kuroha, T., Satoh, S., 2007. Involvement of cytokinins in adventitious and lateral root formation. Plant Root, 1:27–33. [doi:10.3117/plantroot.1.27]
Leonardi, C., Ruggen, A., Malfa, S.I., 2001. Hormone effects on in vitro proliferation and rooting of Grevillea explants. Sci. Hort., 90(3–4):335–341. [doi:10.1016/S0304-4238 (01)00228-X]
Liu, Z.R., Sanford, J.C., 1988. Plant regeneration by organogenesis from strawberry leaf and runner tissue. HortScience, 23:1056–1059.
Ludwig-Müller, J., Vertocnik, A., Town, C.D., 2005. Analysis of indole-3-butyric acid-induced adventitious root formation on Arabidopsis stem segments. J. Exp. Bot., 56(418):2095–2105. [doi:10.1093/jxb/eri208]
Marks, T.R., Simpson, S.E., 2000. Rhizogenesis in Forsythia intermedia and Syringa vulgaris: application of a simple internode experimental system. Plant Cell Rep., 19(12): 1171–1176. [doi:10.1007/s002990000264]
Md Ariff, F.F., Hashim, S.S., Haja, M., Osman, M., 2013. An assessment of genetic relationship among superior accessions of Labisia pumila analyzed by amplified fragment length polymorphism (AFLP) markers. Open Sci. Reposit. Agric., e70081945. [doi:10.7392/Agriculture. 70081945]
Minocha, S.C., 1987. Plant Growth Regulators and Morphogenesis in Cell and Tissue Culture of Forest Trees. In: Cell and Tissue Culture in Forestry. Martinus Nijhoff Publisher, Dordrecht, p.50–66. [doi:10.1007/978-94-017-0994-1_4]
Molina, R.V., Castello, S., Luis, G.A., Guardiola, J.L., 2007. Light cytokinin interaction in shoot formation in epicotyl cuttings of Troyer citrangr cultured in vitro. Plant Cell Tiss. Org. Cult., 89(2–3):131–140. [doi:10.1007/s11240-007-9221-8]
Murashige, T., Skoog, F., 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant., 15(3):473–497. [doi:10.1111/j.1399-3054.1962.tb08052.x]
Nandagopal, S., Kumari, R.B.D., 2007. Effectiveness of auxin induced in vitro root culture in chicory. J. Cent. Eur. Agric., 8(1):73–80.
Nandi, S.K., de Klerk, G.J.M., Parker, C.W., Palni Nandi, L.M.S., 1990. Endogenous cytokinin levels and metabolism of zeatin riboside in genetic tumour tissue and non-tumorous tissues of tobacco. Physiol. Plant., 78(2):197–204. [doi:10.1111/j.1399-3054.1990.tb02081.x]
Nolan, K.E., Irwanto, R.R., Rose, R.J., 2003. Auxin up-regulates MtSERK1 expression in both Medicago truncatula root-forming and embryogenesis cultures. Plant Physiol., 133(1):218–230. [doi:10.1104/pp.103. 020917]
Pérez-Francés, J.F., Valdés, F., Msrtín, R., 1995. Callus induction and culture from explants of Erysimum scoparium in a growth regulator-free medium. Plant Cell Tiss. Org. Cult., 43(3):223–228. [doi:10.1007/BF00039948]
Pierik, R.L.M., Steegmans, H.H.M., 1975. Analysis of adventitious root formation in insolated stem explants of Rhododendron. Sci. Hort., 3(1):1–20. [doi:10.1016/0304-4238(75)90031-X]
Reed, B.M., Esquivel, A.A., 1991. The used of zeatin to initiate in vitro cultures of Vaccinium species and cultivars. HortScience, 26:1320–1322.
Roberts, K., 2007. Auxin. In: Handbook of Plant Science. John Wiley & Sons Ltd., USA, p.352–360.
Rose, R.J., Wang, X.D., Nolan, K.E., Rolfe, B.G., 2006. Root meristems in Medicago truncatula tissue culture arise from vascular-derived procambial-like cells in a process regulated by ethylene. J. Exp. Bot., 57(10):2227–2235. [doi:10.1093/jxb/erj187]
Roy, A.T., De, D.N., 1990. Tissue culture and plant regeneration from immature embryo explants of Calotropis gigantean (L.) R. Br. Plant Cell Tiss. Org. Cult., 20:229–233. [doi:10.1007/BF00041886]
Sahoo, Y., Chand, P.K., 1998. In vitro multiplication of a medicinal herb, Tridax procumbens L. (Mexican daisy, Coatbuttons): influence of explanting season, growth regulator synergy, culture passage and planting substrate. Phytomorphology, 48(2):195–205.
Sreedhar, R.V., Venkatachalam, L., Thimmaraju, R., Bhagyalakshmi, N., Narayan, M.S., Ravishankar, G.A., 2008. Direct organogenesis from leaf explants of Stevia rebaudiana and cultivation in bioreactor. Biol. Plant., 52(2):355–360. [doi:10.1007/s10535-008-0073-9]
Srivastava, L.M., 2001. Auxins. In: Plant Growth and Development: Hormones and Environment. Academic Press, USA, p.155–171.
Su, Y.H., Liu, Y.B., Zhang, X.S., 2011. Auxin-cytokinin interaction regulates meristem development. Mol. Plant, 4(4):616–625. [doi:10.1093/mp/ssr007]
Thomas, C., Meyer, D., Himber, C., Steinmetz, A., 2004. Spatial expression of sunflower SERK gene during induction of somatic embryogenesis and shoot organogenesis. Plant Physiol. Biochem., 42(1):35–42. [doi:10. 1016/j.plaphy.2003.10.008]
Vesperinas, E.S., 1998. In vitro root induction in hypocotyl and plumule explants of Helianthus annuus. Env. Exp. Bot., 39(3):271–277. [doi:10.1016/S0098-8472(98)00019-7]
Wang, B., Peng, D.X., Liu, L.J., Sun, Z.H., Zhang, N., Gao, S.M., 2007. An efficient adventitious shoot regeneration system for ramie (Boehmeria nivea Gaud) using thidiazuron. Bot. Stud., 93:173–180.
Watas, A.A., Ben, J.J., Tal, E., Solomon, H., 1992. In vitro propagation of Grevillea species. Acta Hort., 316:51–54.
Xu, Z., Um, Y.C., Kim, C.H., Lu, G., Guo, D.P., Liu, H.L., Bah, A.A., Mao, A., 2008. Effect of plant growth regulators, temperature and sucrose on shoot proliferation from the stem disc of Chinese jiaotou (Allium chinense) and in vitro bulblet formation. Acta Physiol. Plant, 30(4):521–528. [doi:10.1007/s11738-008-0150-x]
Yamada, Y., Sekiya, J., Koshimizu, K., 1972. Cytokinininduced shoot formation. Phytochemistry, 11(3):1019–1021. [doi:10.1016/S0031-9422(00)88447-2]
Zaizuhana, S., Puteri, J., Noor, M.B., Noral’ashikin, Y., Muhammad, H., Rohana, A.B., Zakiah, I., 2006. The in vivo rodent micronucleus assay of Kacip Fatimah (Labisia pumila) extract. Trop. Biomed., 23(2):214–219.
Zolman, B.K., Martinez, N., Millius, A., Adham, A.R., Bartel, B., 2008. Identification and characterization of arabidopsis indole-3-butyric acid response mutants detective in novel peroxisomal enzymes. Genetics, 180(1):237–251. [doi:10.1534/genetics.108.090399]
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ling, A.P.K., Tan, K.P. & Hussein, S. Comparative effects of plant growth regulators on leaf and stem explants of Labisia pumila var. alata . J. Zhejiang Univ. Sci. B 14, 621–631 (2013). https://doi.org/10.1631/jzus.B1200135
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1200135