Abstract
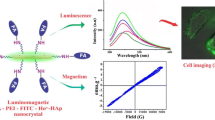
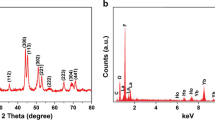
Herein, we report luminomagnetic nanocrystalline hydroxyapatite doped with paramagnetic labels and surface modified with luminescent copper nanoclusters. The dopant concentration is obtained through ICP-MS analysis as 4.99% of Gd3+ and 0.61% of Ho3+. Upon excitation at 310 nm, nanocrystals exhibit luminescence with emission maxima at 402 nm. The paramagnetic nanocrystals show saturation magnetization of 19.063 emu g−1. The nanocrystals have potential in develo** T1 and T2 bimodal MRI contrast agents due to remarkable longitudinal as well as transverse relaxivities, values of which are 12.25 s−1 mM−1 and 24.76 s−1 mM−1 respectively. The MTT assay and morphological examination on HeLa cells revealed non-toxicity of nanocrystals up to 250 µg/mL for 24 h incubation. It is investigated that nanocrystals are readily taken up by L929 mouse lung fibroblast cells. Said nanocrystals are potentially persuasive nanoprobes for bimodal MRI and fluorescence bioimaging.
Graphical abstract










Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its supplementary information files.
References
A. Biedrzycka, E. Skwarek, U.M. Hanna, Hydroxyapatite with magnetic core: synthesis methods, properties, adsorption and medical applications. Adv. Colloid Interface Sci. 291, 102401–102421 (2021). https://doi.org/10.1016/j.cis.2021.102401
M. Du, J. Chen, K. Liu, H. **ng, C. Song, Recent advances in biomedical engineering of nano-hydroxyapatite including dentistry, cancer treatment and bone repair. Compos. B 215, 10790–10812 (2021). https://doi.org/10.1016/j.compositesb.2021.108790
Y. Ryabenkova, N. Jadav, M. Conte, M.F. Hippler, N. Reeves-McLaren, P.D. Coates, P. Twigg, A. Paradkar, Mechanism of hydrogen-bonded complex formation between ibuprofen and nanocrystalline hydroxyapatite. Langmuir 33, 2965–2976 (2017). https://doi.org/10.1021/acs.langmuir.6b04510
S. Panda, C.K. Biswas, S. Paul, A comprehensive review on the preparation and application of calcium hydroxyapatite: a special focus on atomic do** methods for bone tissue engineering. Ceram. Int. 47, 28122–28144 (2021). https://doi.org/10.1016/j.ceramint.2021.07.100
W. Ll, H. Xr, F. Ln, Z. Ym, L. Xx, T. Cy, Drug delivery properties of three-dimensional ordered macroporous zinc-doped hydroxyapatite. J. Mater. Res. 37, 2314–2321 (2022). https://doi.org/10.1557/s43578-022-00632-z
X. Li, D. Zeng, L. Chen, P. Ke, Y. Tian, G. Wang, Preparation and characterization of magnetic chitosan hydroxyapatite nanoparticles for protein drug delivery and antibacterial activity. J. Mater. Res. 36, 4307–4316 (2021). https://doi.org/10.1557/s43578-021-00424-x
D. Kamnoore, D. Mukherjee, D.N. Ammunje, P. Parasuraman, B.V. Teja, M. Radhika, Hydroxyapatite nanoparticle-enriched thiolated polymer-based biocompatible scaffold can improve skin tissue regeneration. J. Mater. Res. 36, 4287–4306 (2021). https://doi.org/10.1557/s43578-021-00405-0
S.S. Syamchand, G. Sony, Multifunctional hydroxyapatite nanoparticles for drug delivery and multimodal molecular imaging. Microchim. Acta 182, 1567–1589 (2015). https://doi.org/10.1007/s00604-015-1504-x
B. Yilmaz, A.Z. Alshemary, Z. Evis, Co-doped hydroxyapatites as potential materials for biomedical applications. Microchem. J. 144, 443–453 (2019). https://doi.org/10.1016/j.microc.2018.10.007
R. Zhou, Y. Li, D. **ao, T. Li, F.W. ZhangT, Y. Lin, Hyaluronan-directed fabrication of co-doped hydroxyapatite as a dual-modal probe for tumor-specific bioimaging. J. Mater. Chem. B 8, 2107–2114 (2020). https://doi.org/10.1039/C9TB02787D
N.L. Ignjatović, L. Mančić, M. Vuković et al., Rare-earth (Gd3+, Yb3+/Tm3+, Eu3+) co-doped hydroxyapatite as magnetic, up-conversion and down-conversion materials for multimodal imaging. Sci. Rep. 9, 16305–16319 (2019). https://doi.org/10.1038/s41598-019-52885-0
M. Liu, H. Liu, Li.X. SunS, H.Z. ZhouY, J. Lin, Multifunctional hydroxyapatite/Na(Y/Gd)F4:Yb3+, Er3+ composite fibers for drug delivery and dual modal imaging. Langmuir 30, 1176–1182 (2014). https://doi.org/10.1021/la500131d
A. Tesch, C. Wenisch, K.-H. Herrmann, J.R. Reichenbach, P. Warncke, D. Fischer, F.A. Müller, Luminomagnetic Eu3+- and Dy3+-doped hydroxyapatite for multimodal imaging. Mater. Sci. Eng. C 81, 422–431 (2017). https://doi.org/10.1016/j.msec.2017.08.032
Y. He, C. Lv, Wu.L. HouX, Mono-dispersed nano-hydroxyapatite based MRI probe with tetrahedral DNA nanostructures modification for in vitro tumor cell imaging. Anal. Chim. Acta 1138, 141–149 (2020). https://doi.org/10.1016/j.aca.2020.09.006
E. Peng, F. Wang, J.M. Xue, Nanostructured magnetic nanocomposites as MRI contrast agents. J. Mater. Chem. B 3, 2241–2276 (2015). https://doi.org/10.1039/C4TB02023E
M. Norek, J.A. Peters, MRI contrast agents based on dysprosium or holmium. Prog. Nucl. Magn. Reson. Spectrosc. 59, 64–82 (2011). https://doi.org/10.1016/j.pnmrs.2010.08.002
Z. Zhou, R. Bai, J. Munasinghe, Z. Shen, L. Nie, X. Chen, T1–T2 dual-modal magnetic resonance imaging: from molecular basis to contrast agents. ACS Nano 11, 5227–5232 (2017). https://doi.org/10.1021/acsnano.7b03075
L. Frullano, T.J. Meade, Multimodal MRI contrast agents. J. Biol. Inorg. Chem. 12, 939–949 (2007). https://doi.org/10.1007/s00775-007-0265-3
Y.K. Peng, C.N. Lui, Y.W. Chen, S.W. Chou, E. Raine, P.T. Chou, K.K. Yung, S.E. Tsang, Engineering of single magnetic particle carrier for living brain cell imaging: a tunable T1-/T2-/dual-modal contrast agent for magnetic resonance imaging application. Chem. Mater. 29, 4411–4417 (2017). https://doi.org/10.1021/acs.chemmater.7b00884
A. Szpak, S. Fiejdasz, W. Prendota et al., T1–T2 Dual-modal MRI contrast agents based on superparamagnetic iron oxide nanoparticles with surface attached gadolinium complexes. J. Nanopart. Res. 16, 2678–2688 (2014). https://doi.org/10.1007/s11051-014-2678-6
M. Yang, L. Gao, K. Liu, C. Luo, Y. Wang, L. Yu, H. Peng, W. Zhang, Characterization of Fe3O4/SiO2/Gd2O(CO3)2 core/shell/shell nanoparticles as T1 and T2 dual mode MRI contrast agent. Talanta 131, 661–665 (2015). https://doi.org/10.1016/j.talanta.2014.08.042
X. Wang, Z. Zhou, Z. Wang, Y. Xue, Y. Zeng, J. Gao, L. Zhu, L.G. ZhangX, X. Chen, Gadolinium embedded iron oxide nanoclusters as T1–T2 dual-modal MRI-visible vectors for safe and efficient siRNA delivery. Nanoscale 5, 8098–8104 (2013). https://doi.org/10.1039/C3NR02797J
O.S. Wolfbeis, An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 44, 4743–4768 (2015). https://doi.org/10.1039/c4cs00392f
H.-S. Peng, D.T. Chiu, Soft fluorescent nanomaterials for biological and biomedical imaging. Chem. Soc. Rev. 44, 4699–4722 (2015). https://doi.org/10.1039/C4CS00294F
ChenF. LiuH, P. **, B. Chen, L. Huang, J. Cheng, W.J. ShaoC, D. Bai, Z. Zeng, Biocompatible fluorescent hydroxyapatite: synthesis and live cell imaging applications. J. Phys. Chem. C 115, 18538–18544 (2011). https://doi.org/10.1021/jp206843w
H. Liu, L. Liao, X. Pan, K. Su, P. Shuai, Z. Yan, Q. Guo, L. Mei, Recent research progress of luminescent materials with apatite structure: a review. Open Ceram. 10, 100251–100266 (2022). https://doi.org/10.1016/j.oceram.2022.100251
Z. Wang, B. Chen, A.L. Rogach, Synthesis, optical properties and applications of light-emitting copper nanoclusters. Nanoscale Horiz. 2, 135–146 (2017). https://doi.org/10.1039/C7NH00013H
Z. Zhao, Y. Li, Develo** fluorescent copper nanoclusters: synthesis, properties, and applications. Colloids Surf. B195, 111244–111253 (2020). https://doi.org/10.1016/j.colsurfb.2020.111244
L. Zhang, E. Wang, Metal nanoclusters: new fluorescent probes for sensors and bioimaging. Nano Today 9, 132–157 (2014). https://doi.org/10.1016/j.nantod.2014.02.010
T.-Q. Yang, B. Peng, B.-Q. Shan et al., Origin of the photoluminescence of metal nanoclusters: from metal-centered emission to ligand-centered emission. Nanomaterials 10, 261 (2020). https://doi.org/10.3390/nano10020261
A. Baghdasaryan, T. Bürgi, Copper nanoclusters: designed synthesis, structural diversity, and multiplatform applications. Nanoscale 13, 6283–6340 (2021). https://doi.org/10.1039/D0NR08489A
Y. An, Y. Ren, M. Bick, A. Dudek, E.H.-W. Waworuntu, J. Tang, J. Chen, B. Chang, Highly fluorescent copper nanoclusters for sensing and bioimaging. Biosens. Bioelectron 154, 112078–112091 (2020). https://doi.org/10.1016/j.bios.2020.112078
M. Ramadurai, G. Rajendran, T.S. Bama, P. Prabhu, K. Kathiravan, Biocompatible thiolate protected copper nanoclusters for an efficient imaging of lung cancer cells. J. Photochem. Photobiol. B: Biol. 205, 111845–111852 (2020). https://doi.org/10.1016/j.jphotobiol.2020.111845
R. Rajamanikandan, A. Aazaad, S. Lakshmipathi, M. Ilanchelian, Glutathione functionalized copper nanoclusters as a fluorescence platform for specific biosensing of cysteine and application in cellular imaging. Microchem. J. 158, 105253–110260 (2020). https://doi.org/10.1016/j.microc.2020.105253
D.-E. Lee, H. Koo, I.-C. Sun, J.H. Ryu, K. Kima, I.C. Kwon, Multifunctional nanoparticles for multimodal imaging and theragnosis. Chem. Soc. Rev. 41, 2656–2672 (2012). https://doi.org/10.1039/C2CS15261D
M. Sadat-Shojai, M.-T. Khorasani, E. Dinpanah-Khoshdargi, A. Jamshidi, Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 9, 7591–7621 (2013). https://doi.org/10.1016/j.actbio.2013.04.012
A. Ashokan, D. Menon, S. Nair, M. Koyakutty, A molecular receptor targeted, hydroxyapatite nanocrystal based multi-modal contrast agent. Biomaterials 31, 2606–2616 (2010). https://doi.org/10.1016/j.biomaterials.2009.11.113
L. **, L. Junkang, Fast synthesis of copper nanoclusters through the use of hydrogen peroxide additive and their application for the fluorescence detection of Hg2+ in water samples. New J. Chem. 39, 5240–5248 (2015). https://doi.org/10.1039/C5NJ00831J
E. Mavropoulos, A.M. Costa, L.T. Costa, C.A. Achete, A. Mello, J.M. Granjeiro, A.M. Rossi, Adsorption and bioactivity studies of albumin onto hydroxyapatite surface. Colloids Surf. B 83, 1–9 (2011). https://doi.org/10.1016/j.colsurfb.2010.10.025
D.T.H. Wassell, R.C. Hall, G. Embery, Adsorption of bovine serum albumin onto hydroxyapatite. Biomaterials 16, 697–702 (1995). https://doi.org/10.1016/0142-9612(95)99697-K
S.K. Swain, D. Sarkar, Study of BSA protein adsorption/release on hydroxyapatite nanoparticles. Appl. Surf. Sci. 286, 99–103 (2013). https://doi.org/10.1016/j.apsusc.2013.09.027
N. Lee, D. Yoo, D. Ling, M.H. Cho, T. Hyeon, J. Cheon, Iron oxide based nanoparticles for multimodal imaging and magnetoresponsive therapy. Chem. Rev. 115, 10637–10689 (2015). https://doi.org/10.1021/acs.chemrev.5b00112
L.H. Reddy, J.L. Arias, J. Nicolas, P. Couvreur, Magnetic nanoparticles: design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 112, 5818–5878 (2012). https://doi.org/10.1021/cr300068p
Acknowledgments
The director of the CSIR-NIIST in Thiruvananthapuram, the head of SAIF at IIT Madras, the director of IISER in Thiruvananthapuram, the director of SAIF-STIC-CUSAT in Kochi, the director of CLIF at the University of Kerala, the head of the chemistry department at the University of Kerala (Kariavattom Campus), and the head of the chemistry department at University College in Thiruvananthapuram are all thanked by the authors for providing advanced characterization techniques and laboratory facilities for the present work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicting interests declared by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Devi, J.S., Surendran, D. & Syamchand, S.S. Magneto-luminescent nanocrystalline hydroxyapatite (Gd, Ho: HAp @Cu-NC) for prospective T1-T2 magnetic resonance imaging and fluorescence bioimaging. Journal of Materials Research 38, 1963–1972 (2023). https://doi.org/10.1557/s43578-023-00933-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/s43578-023-00933-x