Abstract
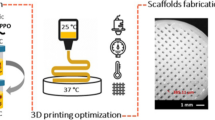
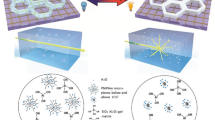
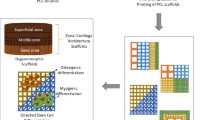
This study investigated a new strategy for fabricating porous scaffolds with the self-folding ability and controlled release of growth factors (GFs) via 3D printing. The scaffolds were a bilayer structure comprising a poly(D,L-lactide-co-trimethylene carbonate) scaffold for providing the shape morphing ability and a gelatin methacrylate scaffold for encapsulating and delivering GF. The structure, shape morphing behavior, GF release, and its effect on stem cell behavior were studied for new scaffolds. The results suggest that these scaffolds have great potential for regenerating tissues such as blood vessels. This work also contributes to developments of 3D printing in tissue engineering.




Similar content being viewed by others
References
C. Wang, Q. Zhao, and M. Wang: Cryogenic 3D printing for producing hierarchical porous and rhBMP-2-loaded Ca-P/PLLA nanocomposite scaffolds for bone tissue engineering. Biofabrication 9, 025031 (2017).
B. Duan and M. Wang: Chapter 4 Selective laser sintering and its biomedical applications. In LaserTechnology in Biomimetics–Basics and Applications, edited by V. Schmidt (Springer, Berlin, 2013), pp. 83–109. ISBN 978-3-642-41340-7.
C.W. Hull: Apparatus for production of three-dimensional objects by stereolithography. U.S. Patent No. 45 753 301, 1986.
S.C. Ligon, R. Liska, J. Stampfl, M. Gurr, and R. Mulhaupt: Polymers for 3D printing and customized additive manufacturing. Chem. Rev. 117, 10212 (2017).
C.K. Chua and W.Y. Yeong: Bioprinting: Principles and Applications, Vol. 1 (World Scientific Publishing Co. Inc., Singapore, 2014).
V.M. Sean and A. Anthony: 3D bioprinting of tissues and organs. Nat. Biotechnol. 32, 773 (2014).
S. Tibbits: The emergence of “4D printing”. In TED Conference, 2013.
Z. Wan, P. Zhang, Y. Liu, L. Lv, and Y. Zhou: Four-dimensional bioprinting: current developments and applications in bone tissue engineering. Acta Biomater. 101, 26–42 (2019).
C. Wang, Y. Zhou, and M. Wang: In situ delivery of rhBMP-2 in surface porous shape memory scaffolds developed through cryogenic 3D plotting. Mater. Lett. 189, 140 (2017).
S. Miao, W. Zhu, N.J. Castro, M. Nowicki, X. Zhou, H. Cui, J.P. Fisher, and L.G. Zhang: 4D printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate. Sci. Rep. 6, 27226 (2016).
M. Zarek, N. Mansour, S. Shapira, and D. Cohn: 4D printing of shape memory-based personalized endoluminal medical devices. Macromol. Rapid Commun. 38, 1600628 (2017).
L. Zhang, Y. **ang, H. Zhang, L. Cheng, X. Mao, N. An, L. Zhang, J. Zhou, L. Deng, and Y. Zhang: A biomimetic 3D-self-forming approach for microvascular scaffolds. Adv. Sci. 1903553 (2020).
G.L. Koons and A.G. Mikos: Progress in three-dimensional printing with growth factors. J. Control. Release 295, 50–59 (2019).
L.K. Narayanan, P. Huebner, M.B. Fisher, J.T. Spang, B. Starly, and R.A. Shirwaiker: 3D-bioprinting of polylactic acid (PLA) nanofiber–alginate hydrogel bioink containing human adipose-derived stem cells. ACS Biomater. Sci. Eng. 2, 1732 (2016).
Y. Zhou, Q. Zhao, and M. Wang: Dual release of VEGF and PDGF from emulsion electrospun bilayer scaffolds consisting of orthogonally aligned nanofibers for gastrointestinal tract regeneration. MRS Commun. 9, 1098 (2019).
M.T. Poldervaart, H. Gremmels, K. van Deventer, J.O. Fledderus, F.C. Öner, M.C. Verhaar, W.J. Dhert, and J. Alblas: Prolonged presence of VEGF promotes vascularization in 3D bioprinted scaffolds with defined architecture. J. Control. Release 184, 58 (2014).
M. Bao, X. Lou, Q. Zhou, W. Dong, H. Yuan, and Y. Zhang: Electrospun biomimetic fibrous scaffold from shape memory polymer of PDLLA-co-TMC for bone tissue engineering. ACS Appl. Mater. Interfaces 6, 2611–2621 (2014).
K. Yue, G. Trujillo-de Santiago, M.M. Alvarez, A. Tamayol, N. Annabi, and A. Khademhosseini: Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 73, 254–271 (2015).
X. Du, H. Cui, B. Sun, J. Wang, Q. Zhao, K. **a, T. Wu, and M.S. Humayun: Photothermally triggered shape-adaptable 3D flexible electronics. Adv. Mater. Technol. 2, 1700120 (2017).
W. Wang, C. Li, M. Cho, and S.H. Ahn: Soft tendril-inspired grippers: shape morphing of programmable polymer–paper bilayer composites. ACS Appl. Mater. Inter faces 10, 10419–10427 (2018).
A.I. Van Den Bulcke, B. Bogdanov, N. De Rooze, E.H. Schacht, M. Cornelissen, and H. Berghmans: Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 1, 31 (2000).
L. Struik: Orientation effects and cooling stresses in amorphous polymers. Polym. Eng. Sci. 18, 799–811 (1978).
H. Li, Y.J. Tan, S. Liu, and L. Li: Three-dimensional bioprinting of oppositely charged hydrogels with super strong interface bonding. ACS Appl. Mater. Interfaces 10, 11164 (2018).
K.Y. Lee and D.J. Mooney: Alginate: properties and biomedical applications. Prog. Polym. Sci. 37, 106 (2012).
J.Y. Park, J.H. Shim, S.A. Choi, J. Jang, M. Kim, S.H. Lee, and D.W. Cho: 3D printing technology to control BMP-2 and VEGF delivery spatially and temporally to promote large-volume bone regeneration. J. Mater. Chem. B 3, 5415 (2015).
W. Zhu, H. Cui, B. Boualam, F. Masood, E. Flynn, R.D. Rao, Z.Y. Zhang, and L.G. Zhang: 3D bioprinting mesenchymal stem cell-laden construct with core–shell nanospheres for cartilage tissue engineering. Nanotechnology 29, 185101 (2018).
Acknowledgments
J.L. and J.L. thank The University of Hong Kong (HKU) for awarding them with research scholarships at HKU. This work was financially supported by Hong Kong Research Grants Council (RGC) through a GRF research grant (17201017) and by HKU through a Seed Fund for Basic Research grant. Assistance provided by members in M. Wang’s group and by technical staff in HKU’s Department of Mechanical Engineering, Faculty of Dentistry and Electron Microscopy Unit is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Supporting Information
Rights and permissions
About this article
Cite this article
Lai, J., Li, J. & Wang, M. 3D Printed porous tissue engineering scaffolds with the self-folding ability and controlled release of growth factor. MRS Communications 10, 579–586 (2020). https://doi.org/10.1557/mrc.2020.65
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1557/mrc.2020.65