Abstract
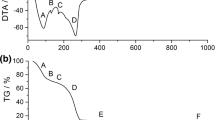
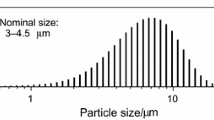
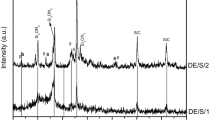
Source material purification according to a thermodynamic analysis is reported for the sublimation crystal growth of aluminum nitride in an inert reactor. OAlOH is strongly favored over all other possible oxygen containing compounds in both the Al-O-H-N and Al-O-H-C-N systems, while Al2O, proved to be the most favorable oxygen containing gas species for Al-O-N system in previous study, become secondary favorable gas species. A low temperature (<1200 °C) treatment is effective in eliminating oxygen and hydrogen from the source powder. Carbon monoxide is another important oxygen containing gas species in the Al-O-H-C-N system, and is favored over Al2O at certain temperature and pressure. Carbothermal reduction with intentionally added carbon (graphite) can further reduce the oxygen concentration. Experiments show that high-temperature sintering minimizes the oxygen concentration and reduces the specific surface area of the source. With only 5.5% of mass loss, the purification produced a source with low O, H, and C concentrations of 0.018 wt%, 6ppm, and 0.006wt%.
Similar content being viewed by others
References
M. Miyanaga, N. Mizuhara, S. Fujiwara, M. Shimazu, H. Nakahata, T. Kawase, J. Crystal Growth 300 (2007) 45.
K. Wafers, C. Bell, Oxides and hydroxides of aluminum, Alcoa Technical Paper no. 19, Alcoa Research Laboratories.
J. Li, M. Nakamyra, T. Shirai, K. Matsumary, C. Ishizaki, and K. Ishizaki, J. Am. Ceram. Soc., 89[3] 937–943 (2006)
M.L. Panchula, J.Y. Ying, J. Am. Ceram. Soc. 86 (2003) 1114.
J. Edgar, L. Du, L. L. Nyakiti, J. Chaudhuri, J. Crystal Growth 310 (2008) 4002–4006
H. Fukuyama, S. Kusunoki, A. Hakomori, and K. Hiraga, J. Appl. Phys. 100, 024905 (2006)
Wataru Nakaoa, Hiroyuki Fukuyama, J. Crystal Growth 259 (2003) 4001–4006, 302-308
NIST-JANAF Thermochemical Tables, 4th ed., M. W. Chase, Jr. Am. Chem. Soc., DC, 1998, Am. Inst. Phys., NY, 1998, the NIST, Gaithersburg, MD 1998.
L. Du and J.H. Edgar, Mater. Res. Soc. Symp. Proc. 955 I11–105 (2007).
Acknowledgments
Support for this project from the II-VI Foundation is greatly appreciated.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Du, L., Edgar, J. Thermodynamic Analysis and Purification for Source Materials in Sublimation Crystal Growth of Aluminum Nitride. MRS Online Proceedings Library 1202, 508 (2009). https://doi.org/10.1557/PROC-1202-I05-08
Published:
DOI: https://doi.org/10.1557/PROC-1202-I05-08