Abstract
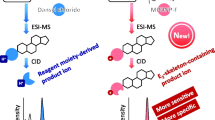
The extensive metabolism and treatment of low doses of estrone (E1), estradiol (E2) and estriol (E3) in preclinical animal species necessitates a sensitive analytical method to identify or quantify the estrogens in biological matrixes. In this study, a highly sensitive and specific method based on the derivatization of E1, E2 and E3 with 10-ethyl-acridine-2-sulfonyl chloride (EASC) coupled with liquid chromatography-ion-trap mass spectrometry with APCI-MS (MRM) identification of estrogens has been developed. The EASC derivatization of E1, E2 and E3 introduces an acridine functional group into estrogen molecules. The carbonyl group in EASC core results in the formation of a phenoxide negative ion by the intramolecular keto-enol isomerization that can be accepted a [H]+ and readily ionized in commonly used LC mobile phases. Derivatives are sufficiently stable to be efficiently analyzed by LC-APCI-MS and show an intense protonated molecular ion at m/z [M+H]+ in positive-ion mode. The collision-induced dissociation of molecular ion forms a distinctive product ion at m/z 222.6, corresponding to the protonated 10-ethyl-acridine moiety. The selected reaction monitoring, based on the m/z [M+H]+ → m/z 222.6 transitions, is highly specific for estrogen derivatives. Therefore, the facile EASC derivatization coupling with LC-APCI-MS analysis allows the development of a highly sensitive and specific method for the identification of trace levels of estrogens in urine of root vole (Microtus oeconomus Pallas).




Similar content being viewed by others
References
Harbuz MS, Lightman SL (1992) J Endocrinol 134:327–339. doi:10.1677/joe.0.1340327
Holst DV (1998) Adv Stud Behav 27:1–131. doi:10.1016/S0065-3454(08)60362-9
Matejicek D, Kuban V (2008) J Chromatogr A 1192:248–253. doi:10.1016/j.chroma.2008.03.061
Sachser N, Dürschlag M, Hirzel D (1998) Psychoneuroendocrino 23:891–904. doi:10.1016/S0306-4530(98)00059-6
Moss AM, Clutton-Brock TH, Monfort SL (2001) Endocrinology 122:158–171. doi:10.1006/gcen.2001.7622
Lopez de Alda MJ, Barcelo D (2001) Fresenius’ J Anal Chem 371:437–447. doi:10.1007/s002160101027
Turner JW, Tolson P, Hamad N (2002) J Zoo Wildl Med 33:214–221
Ren BP, **a SZ, Li QF, Liang B, Qiu JH, Zhang SY (2003) Acta Zool Sin 3:325–331
Good T, Khan MZ, Lynch JW (2003) Physiol Behav 80:405–411. doi:10.1016/j.physbeh.2003.09.006
Goymann W, Möstl E, Van’t Hof T, East ML, Heribert H (1999) Gen Comp Endocrinol 114:340–348. doi:10.1006/gcen.1999.7268
Mateo JM, Cavigelli SA (2005) Physiol Biochem Zool 78:1069–1084. doi:10.1086/432855
Guo T, Gu JH, Soldin OP, Singh RJ, Soldin SJ (2008) Clin Biochem 41:736–741. doi:10.1016/j.clinbiochem.2008.02.009
Cao Z, Swift TA, West CA, Rosano TG, Rej R (2004) Clin Chem 50:160–165. doi:10.1373/clinchem.2003.023325
Diaz-Cruz MS, Lopez de Alda MJ, Lopez R, Barcelo D (2003) J Mass Spectrom 38:917–923. doi:10.1002/jms.529
Baronti C, Curini G, Di Corcia A, Gentili A, Samperi R (2000) Environ Sci Technol 34:5059–5066. doi:10.1021/es001359q
Rodriquez-Mozaz S, de Alda MJL, Barcelo D (2004) Anal Chem 76:6998–7006. doi:10.1021/ac049051v
Isobe T, Shiraishi H, Yasuda M, Shinoda A, Suzuki H, Morita M (2003) J Chromatogr A 984:195–202. doi:10.1016/S0021-9673(02)01851-4
Ingrand V, Herry G, Beausse J, Renee de Roubin M (2003) J Chromatogr A 1020:99–104. doi:10.1016/S0021-9673(03)00770-2
Benijts T, Lambert W, De Leenheer A (2004) Anal Chem 76:704–711. doi:10.1021/ac035062x
Lin YH, Chen CY, Wang GS (2007) Rapid Commun Mass Spectrom 21:1973–1983. doi:10.1002/rcm.3050
Zhang F, Barteks MJ, Brodeur JC, McClymont EL, Woodburn KB (2004) Rapid Commun Mass Spectrom 18:2739–2742. doi:10.1002/rcm.1690
Salvador A, Moretton C, Piram A, Faure R (2007) J Chromatogr A 1145:102–109. doi:10.1016/j.chroma.2007.01.055
Anri MR, Bakhtiar R, Zhu B, Huskey S, Franklin RB, Evans DC (2002) Anal Chem 74:4136–4144. doi:10.1021/ac025712h
Qin F, Zhao Y-Y, Sawyer MB, Li X-F (2008) Anal Chem 80:3404–3411. doi:10.1021/ac702613k
Higashi T, Takayama N, Kyutoku M, Shimada K, Koh E, Namiki M (2006) Steroids 71:1007–1013. doi:10.1016/j.steroids.2006.08.003
Nishiyama T, Hashimoto Y, Takahashi K (2004) Clin Cancer Res 10:7121–7126. doi:10.1158/1078-0432.CCR-04-0913
Yamashita K, Okuyama M, Watanabe Y, Honma S, Kobayashi S, Numazawa M (2007) Steroids 72:819–827. doi:10.1016/j.steroids.2007.07.003
Storoniak P, Krzymiński K, Bouźyk A, Koval’chuk EP, Blaźejowski J (2003) J Therm Anal Calorim 74:443–450. doi:10.1023/B:JTAN.0000005179.91819.6d
Lagana A, Bacaloni A, Fago G, Marino A (2000) Rapid Commun Mass Spectrom 14:401–407. doi:10.1002/(SICI)1097-0231(20000331)14:6<401::AID-RCM883>3.0.CO;2-7
You J, Zhang W, Zhang Q, Zhang L, Yan C, Zhang Y (2002) Anal Chem 74:261–269. doi:10.1021/ac010285d
Kosoy A, Möller C, Perdomo D, Bubis J (2004) J Biochem Mol Biol 37(2):260–267
Chen RF (1968) Anal Biochem 25:412–416. doi:10.1016/0003-2697(68)90116-4
D’Ascenzo G, Di Corcia A, Gentili A, Mancini R, Mastropasqua R, Nazzari M, Samperi R (2003) Sci Total Environ 302:199–209. doi:10.1016/S0048-9697(02)00342-X
Higashi T, Takayama N, Nishio T, Taniguchi E, Shimada K (2006) Anal Bioanal Chem 386:658–665. doi:10.1007/s00216-006-0371-z
Acknowledgments
The authors are grateful to Dr. Honghai Zhang for stimulating discussions throughout the study and thank Hongzhen Liu for excellent technical assistance in the laboratory. This work was supported by the National Science Foundation of China (No. 20075016) and by the 100 Talents Programme of The Chinese Academy of Sciences (No. 328)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
You, J., Zhao, H., Sun, Z. et al. 10-Ethyl-acridine-2-sulfonyl Chloride: A New Derivatization Agent for Enhancement of Atmospheric Pressure Chemical Ionization of Estrogens in Urine. Chroma 70, 45–55 (2009). https://doi.org/10.1365/s10337-009-1101-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1365/s10337-009-1101-4