Abstract
Background
Colorectal cancer (CRC) patients with mismatch repair-deficient/microsatellite instability-high (dMMR/MSI-H) status are conventionally perceived as unresponsive to adjuvant chemotherapy (ACT). The mitochondrial transcription factor A (TFAM) is required for mitochondrial DNA copy number (mtDNA-CN) expression. In light of previous findings indicating that the frequent truncating-mutation of TFAM affects the chemotherapy resistance of MSI CRC cells, this study aimed to explore the potential of mtDNA-CN as a predictive biomarker for ACT efficacy in dMMR CRC patients.
Methods
Levels of MtDNA-CN were assessed using quantitative real-time polymerase chain reaction (qRT-PCR) in a cohort of 308 CRC patients with dMMR comprising 180 stage II and 128 stage III patients. Clinicopathologic and therapeutic data were collected. The study examined the association between mtDNA-CN levels and prognosis, as well as the impact of ACT benefit on dMMR CRC patients. Subgroup analyses were performed based mainly on tumor stage and mtDNA-CN level. Kaplan-Meier and Cox regression models were used to evaluate the effect of mtDNA-CN on disease-free survival (DFS) and overall survival (OS).
Results
A substantial reduction in mtDNA-CN expression was observed in tumor tissue, and higher mtDNA-CN levels were correlated with improved DFS (73.4% vs 85.7%; P = 0.0055) and OS (82.5% vs 90.3%; P = 0.0366) in dMMR CRC patients. Cox regression analysis identified high mtDNA-CN as an independent protective factor for DFS (hazard ratio [HR] 0.547; 95% confidence interval [CI] 0.321–0.934; P = 0.0270) and OS (HR 0.520; 95% CI 0.272–0.998; P = 0.0492). Notably, for dMMR CRC patients with elevated mtDNA-CN, ACT significantly improved DFS (74.6% vs 93.4%; P = 0.0015) and OS (81.0% vs 96.7%; P = 0.0017), including those with stage II or III disease.
Conclusions
The mtDNA-CN levels exhibited a correlation with the prognosis of stage II or III CRC patients with dMMR. Elevated mtDNA-CN emerges as a robust prognostic factor, indicating improved ACT outcomes for stages II and III CRC patients with dMMR. These findings suggest the potential utility of mtDNA-CN as a biomarker for guiding personalized ACT treatment in this population.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The incidence of colorectal cancer (CRC) has consistently risen, resulting in significant societal burdens.1 Primary treatment for stages II and III CRC after surgery involves 5-fluorouracil (5-FU)-based adjuvant chemotherapy (ACT).2,3 However, not all CRC patients derive benefits from ACT.4,5
Approximately 10 to 20% of CRC cases exhibit mismatch repair-deficient/microsatellite instability-high (dMMR/MSI-H) status,6 stemming from DNA MMR gene inactivation (MLH1, MSH2, MSH6, and PMS2).7 Numerous studies have reported diminished or absent responses to fluorouracil-based ACT in dMMR CRC patients versus CRC patients with mismatch repair-proficient/microsatellite-stable/(pMMR/MSS).8,9
Conversely, a survival advantage has been observed for stage III CRC patients with dMMR when treated with a combination of oxaliplatin and fluorouracil.10,11 Nevertheless, some studies argue against the definitive survival benefit of oxaliplatin for dMMR CRC patients.12,13 Despite extensive research, the molecular mechanisms underlying chemotherapy insensitivity in dMMR CRC patients remain unclear. In response to this, the National Comprehensive Cancer Network (NCCN) guidelines currently advocate for sparing ACT for stage II CRC patients with dMMR/MSI-H due to inefficacy, whereas all stage III CRC patients are to receive ACT regardless of MMR status.14 Given the substantial associated costs, toxicity, and inconvenience of inappropriate treatments, there is a pressing need to identify predictive biomarkers for ACT efficacy in CRC patients with dMMR.
Mitochondrial DNA copy number (mtDNA-CN), a marker of mitochondrial function,15 is linked to development of chemoresistance in various malignancies.16,17 Prior studies have indicated that decreased mtDNA-CN contributes to chemotherapy resistance in ovarian cancer and esophageal squamous cell carcinoma.17,18 Moreover, human MSI-H CRC frequently exhibits truncating mutations in mitochondrial transcription factor A (TFAM), impairing mitochondrial stability and augmenting cisplatin-dependent apoptotic resistance.19 As TFAM regulates mtDNA-CN expression, TFAM-truncating mutations result in decreased mtDNA-CN expression.19,20 Therefore, it is hypothesized that mtDNA-CN could function as a predictive biomarker for ACT efficacy in dMMR CRC patients.
Based on this, we conducted the current study to assess the impact of mtDNA-CN on prognosis and ACT efficacy in dMMR CRC patients. To our knowledge, this report marks the first instance of mtDNA-CN proposed as an efficacy-predictive biomarker for ACT treatment of dMMR CRC patients.
Methods
Patient Samples
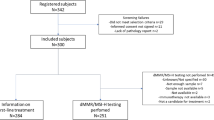
This study collected or obtained 308 CRC specimens with dMMR tissue, including 180 stage II cases and 128 stage III cases, from the tumor tissue bank of the hospital. The selection criteria specified CRC patients who underwent radical resection and patients with pathologic staging of stage II or III disease identified as dMMR by immunohistochemistry (IHC). The exclusion criteria ruled out patients who had local excisions performed, patients who had a history of malignant tumors, and patients with multiple primary CRCs.
The clinicopathologic information and follow-up data of the patients came from the hospital database. The research contents and procedures were approved by the Institutional Review Committee of the Sixth Affiliated Hospital of Sun Yat-sen University (project no. E2021156). All patient information was treated anonymously and confidentially, so informed consent was waived. The analysis process is shown in Fig. 1A.
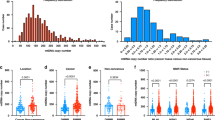
Research flow chart and mtDNA-CN characteristics of stages II and III CRC patients with dMMR. A Schematic diagram of research flow. B The distribution of mtDNA-CN in CRC cases. C The deficient mismatch repair proteins in 308 locally advanced colorectal cancer patients. D The mtDNA-CN ratio in tumor tissue versus non-tumor tissues cases. MtDNA-CN, mitochondrial DNA copy number; CRC, colorectal cancer; dMMR, deficient mismatch repair; IHC, immunohistochemistry; PMMR, mismatch repair-proficient; qPT-PCR, quantitative real-time polymerase chain reaction; ACT, adjuvant chemotherapy; T, tumor; N, non-cancerous
Long-term follow-up evaluation was performed every 6 months for all the patients by telephone or re-examination, and their relevant information was recorded. The main evaluation methods included clinical physical examination, imaging examination, and carcinoembryonic antigen (CEA) detection. In addition, it was recommended that the patients receive computed tomography and colonoscopy every year. Disease-free survival (DFS) was defined as the duration from radical resection to the last follow-up date or tumor recurrence, and overall survival (OS) was defined as the duration from radical resection to the last follow-up date or death from any cause. These follow-up evaluations continued until the death of the patient or the end of the study follow-up period (May 2023).
DNA Isolation
The DNA isolation kit (Omega, Norcrosss, GA, USA) was used to extract DNA from tumor and normal tissues. The specific experimental steps were carried out according to the method provided in the kit protocol, and 30 mg of tissue was cut from each specimen for genomic DNA separation. Moreover, the purity of DNA samples was assessed by Nanodrop technology, and unqualified DNA samples were re-extracted. Finally, all qualified DNA samples were included for the next mtDNA-CN analysis.
Determination of mtDNA-CN
For the determination of mtDNA-CN, quantitative real-time polymerase chain reaction (qPT-PCR) was performed using the Applied Biosystems 7500 Fast Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA). According to the qPT-PCR protocol of TB Green Premix Ex Taq II (TaKaRa, Shiga, Japan), the DNA input of each sample was 100 ng, and each sample was measured three times to ensure reliability of the results. The mean value was used as the mtDNA-CN score for each individual. The primer sequences used were hemoglobin (HGB)-F, 5′-GTGCACCTGACTCCTGAGGAGA-3′ and HGB-R, 5′-CCTTGATACCAACCTGCCCAG-3′ for hemoglobin (nuclear DNA control) and ND1-F, 5′-CCCTAAAACCCGCCACATCT-3′ and ND1-R, 5′-GAGCGATGGTGAGAGCTAAGGT-3′ for mitochondrial NADH dehydrogenase 1, respectively. The qRT-PCR reactions were performed at 95 °C for 30 s followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. Based on the threshold cycle value (Ct values), the MtDNA-CN of each sample was calculated by the 2-ΔCT method.
Treatment
For the patients who received neoadjuvant chemoradiotherapy, from Monday to Friday, the tumor and entire pelvis were treated with radiotherapy (23–25 doses), for a total dose of 46.0 to 50.4 Gy. The patients received preoperative therapy with four to six cycles of fluorouracil (folinate 400 mg/m2 intravenous infusion, fluorouracil 400 mg/m2 intravenous infusion, fluorouracil 2.4 g/m2 intravenous continuous infusion for 48 h) and oxaliplatin 85 mg/m2 intravenously each chemotherapy treatment cycle, as well as radiotherapy during the two to four cycles of chemoradiotherapy. After surgery, six to eight cycles of mFOLFOX6 chemotherapy were administered to patients who required ACT, with additional radiotherapy at the physician's discretion.
Pathologic Assessment
Two experienced pathologists were responsible for the relevant evaluation of surgical pathologic specimens. Immunohistochemistry (IHC) was used to evaluate the MMR status of surgical pathologic specimens. According to the guidelines of NCCN and the Chinese Society of Clinical Oncology, if the expression of MMR gene-related proteins (MLH1, MSH2, MSH6, and PMS2) are lacking in immunohistochemical detection, it will be defined as dMMR. The MMR protein deficiency of patients is shown in Fig. 1C. When the IHC result was uncertain, it was further confirmed by the microsatellite instability (MSI) test based on PCR. The pathologic staging of the CRC patients was determined according to the International Union Against Cancer tumor-node-metastasis (TNM) staging/American Joint Committee on Cancer staging.
Statistical Analysis
GraphPad Prism 9 (San Diego, CA, USA) and SPSS 25.0 (New York, NY, USA) were used for data analysis in this study. The analysis results showed that the distribution of mtDNA-CN was skewed toward the right (Shapiro-Wilk’s W, 0.9231; P < 0.0001), which did not conform to the normal distribution (Fig. 1B). Therefore, we adopted non-parametric statistical test methods for data analysis. The Mann-Whitney’s U test and the Kruskal-Wallis test were used to analyze the relationship between mtDNA-CN and clinicopathologic features where applicable. The chi-square test was used to analyze the classification parameters, and the number and percentage of cases were recorded.
For comparison of continuous variables and means, the Mann-Whitney U test or Student’s t test was used. Regarding survival-related data, the Kaplan-Meier log-rank test was used to compare the effects of various factors on the DFS and OS of the patients. Moreover, the potential prognostic factors were analyzed by the univariate Cox model, and the statistically significant parameters were further determined by the multivariate Cox model. In view of the interaction between indicators and ACT, the Cox proportional hazards regression model was used. The output results included hazard ratios (HRs) and 95% confidence interval (CIs). For all data analysis results, when the two-sided P value was lower than 0.05, we considered the difference as statistically significant.
Results
Characteristics of the CRC Patients With the dMMR Cohort
This study encompassed 308 eligible CRC patients, comprising 180 with stage II and 128 with stage III disease. The age of the participants ranged from 17 to 89 years (median, 54 years). Among the analyzed cohort, 195 (63.3%) were male, and 113 (36.7%) were female. After surgery, 197 patients (64.0%) received ACT, whereas 111 patients (36.0%) did not. During a median follow-up period of 80 months, 63 patients (20.5%) experienced disease recurrence, and 42 patients (13.6%) succumbed to CRC. The clinicopathologic characteristics and treatment details for the cohort of CRC patients with dMMR are presented in Table 1.
Correlation of mtDNA-CN With Clinicopathologic Features and Prognosis
Subsequently, we performed an analysis of the distribution and prognostic implications of mtDNA-CN. Notably, mtDNA-CN exhibited a statistically significant reduction in tumor tissues compared with non-cancerous tissues (P < 0.0001; Fig. 1D). Specifically, an elevation in mtDNA-CN was prominently observed in cases classified as stage T4 (P = 0.0350; Fig. 2D),<F2> exhibiting poor tumor differentiation (P = 0.0363; Fig. 2H) and manifesting nervous infiltration (P = 0.0383; Fig. 2J). Conversely, no statistically significant associations were identified between mtDNA-CN and variables such as sex (P = 0.4429), age (P = 0.2670), tumor location (P = 0.7793), stage N (P = 0.7598), CEA (P = 0.1050), carbohydrate antigen 19-9 (CA19-9) (P = 0.5747), vascular invasion (P = 0.9856), tissue type (P = 0.5789), KRAS status (P = 0.3157), BRAF status (P = 0.1046), NRAS status (P = 0.2400), and PIK3CA status (P = 0.9533). Furthermore, we observed a correlation between low mtDNA-CN and unfavorable disease-free survival (DFS) (73.4% vs 85.7%, P = 0.0055) and overall survival (OS) (82.5% vs 90.3%, P = 0.0366) in the CRC patients with dMMR (Fig. 3A and B).
Correlations between mtDNA-CN and clinicopathologic features in stages II and III CRC with dMMR. A Sex. B Age. C Tumor location. D Stage T. E Stage N. F CEA. G CA19-9. H Tumor differentiation. I Vascular invasion. J Nervous infiltration. K Tissue type. L KRAS status. M BRAF status. N NRAS status. O PIK3CA status. MtDNA-CN, mitochondrial DNA copy number; CRC, colorectal cancer; dMMR, deficient mismatch repair; CEA, carcinoembryonic antigen; CA 19-9, carbohydrate antigen 19-9; AC, adenocarcinoma; MC, mucinous adenocarcinoma; WT, wild type; MT, mutant type
Correlation between ACT, mtDNA-CN and the prognosis of stages II and III CRC patients with dMMR. A Kaplan-Meier analysis of DFS based on mtDNA-CN in the CRC cohort. B Kaplan-Meier analysis of OS based on mtDNA-CN in the CRC cohort. C Kaplan-Meier analysis of DFS based on ACT in the CRC cohort. D Kaplan-Meier analysis of OS based on ACT in the CRC cohort. E Kaplan-Meier analysis of DFS based on ACT in the stage II CRC with dMMR cohort. F Kaplan-Meier analysis of OS based on ACT in the stage II CRC with dMMR cohort. G Kaplan-Meier analysis of DFS based on ACT in the stage III CRC with dMMR cohort. H Kaplan-Meier analysis of OS based on ACT in the stage III CRC with dMMR cohort. ACT, adjuvant chemotherapy; MtDNA-CN, mitochondrial DNA copy number; CRC, colorectal cancer; dMMR, deficient mismatch repair; DFS, disease-free survival; OS, overall survival
Prognostic Analysis
To elucidate the prognostic significance of mtDNA-CN, Cox regression analysis was performed on all the CRC patients with dMMR. Univariate analysis showed that age older than 65 years, pathologic T stage 4, positive pathologic N stage, lymphovascular infiltration, and nervous invasion were associated with poorer DFS for the CRC patients with dMMR. Additionally, elevated mtDNA-CN emerged as a favorable factor for DFS. In the multivariate analysis, age older than 65 years (HR 2.073, 95% CI 1.233–3.488; P = 0.0059), lymphovascular infiltration (HR 2.360; 95% CI 1.320–4.217; P = 0.0037), nervous invasion (HR 2.557; 95% CI 1.327–4.926; P = 0.0050), and high mtDNA-CN (HR 0.547; 95% CI 0.321–0.934; P = 0.0270) were identified as independent factors influencing DFS (Table S1).
Furthermore, the univariate analysis demonstrated that age older than 65 years, pathologic T stage 4, positive pathologic N stage, lymphovascular infiltration, and nervous invasion were correlated with poorer OS. In addition, elevated mtDNA-CN emerged as a favorable factor for OS. The multivariate analysis confirmed that age older than 65 years (HR 3.634; 95% CI 1.958–6.745; P < 0.0001) and high mtDNA-CN (HR 0.520; 95% CI 0.272–0.998; P = 0.0492) were independent factors influencing OS (Table S2).
The Role of Adjuvant Chemotherapy for the CRC Patients With dMMR
Regarding the impact of ACT on the prognosis for the CRC patients with dMMR, the utilization of ACT exhibited a significant enhancement in DFS (73.0% vs 83.2%; P = 0.0483), whereas it did not demonstrate a statistically significant effect on OS (82.0% vs 88.8%; P = 0.1481) (Fig. 3C and D). In the subgroup analysis focused on stage II CRC patients with dMMR, ACT did not yield a significant impact on DFS (79.7% vs 90.1%; P = 0.0649) or OS (84.8% vs 93.1%; P = 0.1028) (Fig. 3E and F). Conversely, for the stage III CRC patients with dMMR, ACT significantly improved DFS (56.3% vs 76.0%; P = 0.0307), although it did not exert a significant effect on OS (75.0% vs 84.4%; P = 0.2311) (Fig. 3G and H). Cox regression analysis indicated that in the broader context of all the CRC patients with dMMR, ACT did not emerge as a significant prognostic factor for DFS (HR 0.611; 95% CI 0.373–1.002; P = 0.0510) or OS (HR 0.642; 95% CI 0.350–1.177; P = 0.1518) (Tables S1 and S2).
MtDNA-CN as a Prognostic Biomarker for the Efficacy of Adjuvant Chemotherapy
We further investigated the potential of mtDNA-CN as a predictive biomarker for the efficacy of ACT for patients with dMMR. Among the dMMR CRC patients with low mtDNA-CN, ACT demonstrated no significant impact on DFS (70.8% vs 74.5%; P = 0.6895) or OS (83.3% vs 82.1%; P = 0.6585) (Fig. 4A and B). Conversely, for the dMMR CRC patients with high mtDNA-CN, ACT significantly improved both DFS (74.6% vs 93.4%; P = 0.0015) and OS (81.0% vs 96.7%; P = 0.0017) (Fig. 4C and D).
A subgroup analysis of the correlation between ACT, mtDNA-CN, and the prognosis of stages II and III CRC patients with dMMR. A Kaplan-Meier analysis of DFS based on ACT in the low mtDNA-CN CRC cohort. B Kaplan-Meier analysis of OS based on ACT in the low mtDNA-CN CRC cohort. C Kaplan-Meier analysis of DFS based on ACT in the high mtDNA-CN CRC cohort. D Kaplan-Meier analysis of OS based on ACT in the high mtDNA-CN CRC cohort. E Kaplan-Meier analysis of DFS based on ACT in the stage II dMMR CRC with low mtDNA-CN cohort. F Kaplan-Meier analysis of OS based on ACT in the stage II dMMR CRC with low mtDNA-CN cohort. G Kaplan-Meier analysis of DFS based on ACT in the stage II dMMR CRC with high mtDNA-CN cohort. H Kaplan-Meier analysis of OS based on ACT in the stage II dMMR CRC with high mtDNA-CN cohort. I Kaplan-Meier analysis of DFS based on ACT in the stage III dMMR CRC with low mtDNA-CN cohort. J Kaplan-Meier analysis of OS based on ACT in the stage III dMMR CRC with low mtDNA-CN cohort. K Kaplan-Meier analysis of DFS based on ACT in the stage III dMMR CRC with high mtDNA-CN cohort. L Kaplan-Meier analysis of OS based on ACT in the stage III dMMR CRC with high mtDNA-CN cohort. ACT, adjuvant chemotherapy; MtDNA-CN, mitochondrial DNA copy number; CRC, colorectal cancer; dMMR, deficient mismatch repair; DFS, disease-free survival; OS, overall survival
To assess whether staging influences outcomes, we conducted subgroup analyses of both staging and mtDNA-CN. For the stage II dMMR CRC patients with low mtDNA-CN, ACT had no discernible effect on DFS (78.4% vs 85.2%; P = 0.5051) or OS (86.5% vs 87.0%; P = 0.9081) (Fig. 4E and F). In contrast, for the stage II dMMR CRC patients with high mtDNA-CN, ACT significantly improved both DFS (81.0% vs 95.7%; P = 0.0112) and OS (83.3% vs 100.0%; P = 0.0050) (Fig. 4G and H).
Similarly, for the stage III dMMR CRC patients with high mtDNA-CN, ACT significantly enhanced both DFS (57.9% vs 88.9%; P = 0.0141) and OS (73.7% vs 95.6%; P = 0.0112) (Fig. 4K and L). Conversely, for the stage III dMMR CRC patients with low mtDNA-CN, ACT had no apparent effect on DFS (53.8% vs 64.7%; P = 0.2821) or OS (76.9% vs 74.5%; P = 0.8473) (Fig. 4I and J).
In addition, subgroup analyses for tumor location showed that tumor location did not appear to influence these findings (Fig. S1). Given the limited subgroup sample size, the results need to be analyzed with caution.
Notably, inherent differences in baseline characteristics exist between patients with and without ACT. To delve deeper into the relationship between mtDNA-CN and ACT, we performed subgroup analyses on all the dMMR CRC patients who received ACT. These analyses showed that for all the dMMR CRC patients with ACT, those who had high mtDNA-CN exhibited superior DFS (73.5% vs 92.9%; P = 0.0003) and OS (80.6% vs 97.0%; P = 0.0001) (Fig. S2A and B).<SF2> Additionally, among the stage II dMMR CRC patients with ACT, those with high mtDNA-CN showed improved DFS (84.0% vs 96.1%; P = 0.0400) and OS (88.0% vs 98.0%; P = 0.0423) (Fig. S2C and D). Similarly, among the stage III dMMR CRC patients with ACT, those with high mtDNA-CN also demonstrated enhanced DFS (62.5% vs 89.6%; P = 0.0030) and OS (72.9% vs 95.8%; P = 0.0008) (Fig. S2E and F).
Predictive Effect of Various Factors on the Efficacy of Adjuvant Chemotherapy
To assess the superiority of mtDNA-CN over other clinicopathologic factors in predicting the efficacy of ACT for CRC patients with dMMR, all relevant factors were evaluated. Regarding DFS, ACT conferred survival benefit for the dMMR CRC patients with positive pathologic N stage (HR 0.490; 95% CI 0.252–0.952; P = 0.0353), poor tumor differentiation (HR 0.474; 95% CI 0.270–0.831; P = 0.0092), and high mtDNA-CN (HR 0.246; 95% CI 0.096–0.629; P = 0.0034) (Fig. 5). Regarding OS, ACT conferred a survival benefit for the dMMR CRC patients with poor tumor differentiation (HR 0.494; 95% CI 0.252–0.969; P = 0.0402) and high mtDNA-CN (HR 0.168; 95% CI 0.048–0.597; P = 0.0058) (Fig. 5). These results suggest that among all clinicopathologic factors, high mtDNA-CN stands out as the most effective predictor of ACT efficacy.
Efficacy of ACT on each clinicopathologic factor and mtDNA-CN for DFS and OS for stages II and III CRC patients with dMMR. ACT, adjuvant chemotherapy; MtDNA-CN, mitochondrial DNA copy number; DFS, disease-free survival; OS, overall survival; CRC, colorectal cancer; dMMR, deficient mismatch repair; CEA, carcinoembryonic antigen; CA 19-9, carbohydrate antigen 19-9
Discussion
This study represents the inaugural disclosure of mtDNA-CN as a potential predictive biomarker for the efficacy of ACT for CRC patients with dMMR. Specifically, for the stage II/III dMMR CRC patients with elevated mtDNA-CN, the administration of ACT significantly improved both DFS and OS. This discovery lays a crucial foundation for tailoring individualized ACT treatment strategies for CRC patients with dMMR, highlighting the significant role of mtDNA-CN in guiding treatment decisions.
In recent years, there has been a growing consensus among medical professionals that MMR status serves as a practical biomarker to inform postoperative treatment decisions in advanced CRC.21 The current NCCN guidelines recommend against ACT for stage II CRC patients with dMMR/MSI-H while advocating for ACT for stage III CRC patients with dMMR/MSI-H.14 This approach is considered relatively safe due to ongoing debates surrounding the use of ACT for CRC patients with dMMR. Numerous clinical data indicate a potential diminished response to fluorouracil in dMMR CRC patients.8,9 Furthermore, fluorouracil-based ACT may even compromise the survival of CRC patients with dMMR.22
These clinical findings align with in vitro experimental results, which demonstrate increased resistance of MSI CRC cells to fluorouracil.23 In fact, a competent MMR system is crucial for the efficacy of fluorouracil because MMR proteins promote DNA damage-induced apoptosis.24 Therefore, it has been suggested that CRC patients with dMMR should not undergo adjuvant fluorouracil therapy. However, this conclusion is not universally accepted, and several studies have reported inconclusive findings regarding the benefits or harms of ACT for CRC patients with dMMR.25,26
In contrast, the addition of oxaliplatin to fluorouracil may confer a survival advantage for stage III CRC patients with dMMR.11,21 A study by Shaib et al.27 demonstrated a significant OS benefit for stage III CRC patients with dMMR who received ACT, particularly with doublet chemotherapy. Retrospective analyses from various clinical trials have indicated that folinic acid-fluorouracil-oxaliplatin (FOLFOX) treatment surpasses fluorouracil alone in efficacy for dMMR CRC.28,29 Preclinical data have similarly indicated that MSI tumor cell lines exhibit sensitivity to oxaliplatin.30 Unlike fluorouracil, oxaliplatin achieves its chemotherapeutic effect through the formation of platinum-DNA adducts, causing DNA damage, including mtDNA, leading to apoptosis, a process not recognized by the MMR system.31,32 However, Müller et al.’s12 findings suggested that MSI-H metastatic CRC patients were less responsive than MSS patients to 5-fluorouracil/folinic acid plus oxaliplatin (FUFOX) or capecitabine plus oxaliplatin (CAPOX) regimens, implying a potential resistance of dMMR status to oxaliplatin-based chemotherapy. Conversely, Kim et al.13 argued that MMR status has no significant impact on the prognosis for patients who receive oxaliplatin-based chemotherapy.13
In summary, the therapeutic efficacy of ACT for CRC patients with dMMR exhibits variability, indicating treatment response heterogeneity in this subgroup. Consequently, sensitivity to ACT may vary among CRC patients with dMMR, warranting the need for individualized treatment due to cost implications, concomitant toxicity, and limited survival benefit.
Identifying a novel biomarker for predicting the response of CRC patients with dMMR to ACT is crucial. Notably, prior studies by Duval’s team suggested that the large deletion of HSP110 T17 correlated with ACT efficacy, either with fluorouracil alone or combined with oxaliplatin, in CRC patients with dMMR.33 Regrettably, this effect was not consistently observed in subsequent studies.34,35
Another investigation by Ryan et al.36 explored the impact of COX2 on the ACT response in CRC patients with dMMR but found no discernible relationship. Our study, in contrast, showed the adverse prognosis associated with low mtDNA-CN in CRC patients with dMMR. Importantly, our results provide evidence that mtDNA-CN levels may help identify a subset of stage II or III CRC patients with dMMR who could benefit from ACT, suggesting mtDNA-CN as a potential predictive biomarker for ACT efficacy.
As a potential biomarker, mtDNA-CN has demonstrated associations with various diseases and cancers.37 Additionally, mitochondrial dysfunction, resulting from genetic changes in mtDNA, has been closely linked to chemoresistance.17,19 In ovarian cancer, depleted mtDNA-CN suppresses production of reactive oxygen species, leading to cisplatin insensitivity.19 Furthermore, in esophageal squamous cancer cells, low mtDNA-CN induces resistance to cisplatin, fluorouracil, and docetaxel chemotherapy via epithelial-mesenchymal transition.18 Previous studies also have indicated that MSI-H CRC with a high frequency of TFAM-truncating mutations demonstrates insensitivity to cisplatin-induced apoptosis due to increased cytochrome b expression.20 Therefore, these findings collectively suggest that mtDNA-CN holds promise as a predictive biomarker for ACT efficacy. From a clinical perspective, the mtDNA-CN test based on qRT-PCR can be performed easily in most hospital laboratories and is cost-effective.
Admittedly, this study had some limitations. First, due to the retrospective design of the study, the lack of some clinical information may have affected the richness and completeness of the results. Second, the patient cohort was not continuously collected, and the sample size was relatively limited, which may have affected the representativeness of the research results. Third, due to the limited sample size, the independent analysis results of colon cancer and rectal cancer could not present potential differences well. Fourth, the predictive efficacy of mtDNA-CN lacks external verification, which limits the generalization of the research results. Finally, this study was conducted within a particular demographic and genetic background, which might limit the applicability of the findings to broader populations. Therefore, we hope that more rigorous prospective studies will be conducted in the future to verify the predictive effect of mtDNA-CN on the acceptance of ACT for CRC patients with dMMR, so the purpose of “seeking benefits and avoiding harms” can be achieved.
Conclusion
In conclusion, our study presents compelling evidence supporting the potential utility of mtDNA-CN levels for identifying a subset of stage II/III CRC patients with dMMR who could derive benefits from ACT. This insight underscores the significance of personalized treatment strategies. The assessment of mtDNA-CN through qRT-PCR emerges as a straightforward and feasible method, offering practical applicability in clinical settings.
Data Availability
All the data are available from the corresponding author on reasonable request.
References
**e Y, Shi L, He X, Luo Y. Gastrointestinal cancers in China, the USA, and Europe. Gastroenterol Rep Oxford. 2021;9:91–104.
Tong M, et al. Multi-omics landscapes of colorectal cancer subtypes discriminated by an individualized prognostic signature for 5-fluorouracil-based chemotherapy. Oncogenesis. 2016;5:e242.
Cai J, et al. ABIN-1 is a key regulator in RIPK1-dependent apoptosis (RDA) and necroptosis, and ABIN-1 deficiency potentiates necroptosis-based cancer therapy in colorectal cancer. Cell Death Dis. 2021;12:140.
Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol. 2010;7:153–62.
Li Y, et al. Immunohistochemistry-based consensus molecular subtypes as a prognostic and predictive biomarker for adjuvant chemotherapy in patients with stage II colorectal cancer. Oncologist. 2020;25:e1968–79.
Peltomäki P. Role of DNA mismatch repair defects in the pathogenesis of human cancer. J Clin Oncol. 2003;21:1174–9.
Baretti M, Le DT. DNA mismatch repair in cancer. Pharmacol Ther. 2018;189:45–62.
Sun BL. Current microsatellite instability testing in management of colorectal cancer. Clin Colorectal Cancer. 2021;20:e12–20.
Sinicrope FA, et al. DNA mismatch repair status and colon cancer recurrence and survival in clinical trials of 5-fluorouracil-based adjuvant therapy. J Natl Cancer Inst. 2011;103:863–75.
Zaanan A, et al. Role of deficient DNA mismatch repair status in patients with stage III colon cancer treated with FOLFOX adjuvant chemotherapy: a pooled analysis from 2 randomized clinical trials. JAMA Oncol. 2018;4:379–83.
Zaanan A, Taieb J. Predictive and prognostic value of MSI phenotype in adjuvant colon cancer: who and how to treat? Bull Cancer. 2019;106:129–36.
Müller CI, et al. Predictive and prognostic value of microsatellite instability in patients with advanced colorectal cancer treated with a fluoropyrimidine and oxaliplatin containing first-line chemotherapy: a report of the AIO Colorectal Study Group. Int J Colorectal Dis. 2008;23:1033–9.
Kim ST, et al. The effect of DNA mismatch repair (MMR) status on oxaliplatin-based first-line chemotherapy as in recurrent or metastatic colon cancer. Med Oncol. 2010;27:1277–85.
Benson AB, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw. 2021;19:329–59.
Abd Radzak SM, et al. Insights regarding mitochondrial DNA copy number alterations in human cancer (review). Int J Mol Med. 2022;50:104
Gonzalez-Sanchez E, Marin JJ, Perez MJ. The expression of genes involved in hepatocellular carcinoma chemoresistance is affected by mitochondrial genome depletion. Mol Pharm. 2014;11:1856–68.
Kubo Y, et al. Low mitochondrial DNA copy number induces chemotherapy resistance via epithelial-mesenchymal transition by DNA methylation in esophageal squamous cancer cells. J Transl Med. 2022;20:383.
Kleih M, et al. Direct impact of cisplatin on mitochondria induces ROS production that dictates cell fate of ovarian cancer cells. Cell Death Dis. 2019;10:851.
Guo J, et al. Frequent truncating mutation of TFAM induces mitochondrial DNA depletion and apoptotic resistance in microsatellite-unstable colorectal cancer. Cancer Res. 2011;71:2978–87.
Venit T, et al. Positive regulation of oxidative phosphorylation by nuclear myosin 1 protects cells from metabolic reprogramming and tumorigenesis in mice. Nat Commun. 2023;14:6328.
Taieb J, et al. Deficient mismatch repair/microsatellite unstable colorectal cancer: diagnosis, prognosis, and treatment. Eur J Cancer. 2022;175:136–57.
Sargent DJ, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–26.
Carethers JM, et al. Mismatch repair proficiency and in vitro response to 5-fluorouracil. Gastroenterology. 1999;117:123–31.
Meyers M, et al. DNA mismatch repair-dependent response to fluoropyrimidine-generated damage. J Biol Chem. 2005;280:5516–26.
Hutchins G, et al. Value of mismatch repair, KRAS, and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer. J Clin Oncol. 2011;29:1261–70.
Klingbiel D, et al. Prognosis of stage II and III colon cancer treated with adjuvant 5-fluorouracil or FOLFIRI in relation to microsatellite status: results of the PETACC-3 trial. Ann Oncol. 2015;26:126–32.
Shaib WL, et al. Survival outcome of adjuvant chemotherapy in deficient mismatch repair stage III colon cancer. Cancer. 2020;126:4136–47.
André T, et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol. 2015;33:4176–87.
Sinicrope FA, et al. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J Clin Oncol. 2013;31:3664–72.
Fink D, et al. The role of DNA mismatch repair in platinum drug resistance. Cancer Res. 1996;56:4881–6.
Qu X, et al. Loss of cancer-associated fibroblast-derived exosomal DACT3-AS1 promotes malignant transformation and ferroptosis-mediated oxaliplatin resistance in gastric cancer. Drug Resist Updat. 2023;68:100936.
Tang Q, et al. Periodic oxaliplatin administration in synergy with PER2-mediated PCNA transcription repression promotes chronochemotherapeutic efficacy of OSCC. Adv Sci Weinh. 2019;6:1900667.
Collura A, et al. Patients with colorectal tumors with microsatellite instability and large deletions in HSP110 T17 have improved response to 5-fluorouracil–based chemotherapy. Gastroenterology. 2014;146:401-411.e401.
Kim JH, et al. Expression status of wild-type HSP110 correlates with HSP110 T17 deletion size and patient prognosis in microsatellite-unstable colorectal cancer. Mod Pathol. 2014;27:443–53.
Tachon G, et al. HSP110 as a diagnostic but not a prognostic biomarker in colorectal cancer with microsatellite instability. Front Genet. 2021;12:769281.
Ryan ÉJ, et al. Effects of CDX2 on prognosis and chemotherapy responsiveness in mismatch repair-deficient colorectal cancer. BJS Open. 2018;2:456–63.
Filograna R, Mennuni M, Alsina D, Larsson NG. Mitochondrial DNA copy number in human disease: the more the better? FEBS Lett. 2021;595:976–1002.
Acknowledgment
This study was supported by the xcience and technology key research and development plan project of Guangzhou (China) (202103000072), the National Natural Science Foundation of China (No. 82170678), the interdisciplinary program of the Wuhan National High Magnetic Field Center (Grant No. WHMFC202113), the Huazhong University of Science and Technology (the Fundamental Research Funds for the Central Universities [HUST (2021JYCXJJ063]), and the 2021 Clinical Research Foundation of Wuhan Union Hospital (F016020042200101506).
Author information
Authors and Affiliations
Contributions
KLC and LH designed the study as the principal investigator. MC collected relevant information and carried out experiments. MC, SHD and YHC interpreted the results and wrote the manuscript draft. JW, FLZ, JNG, FWM and YFX performed the analysis. ZXJ, DLC, NH performed acquisition, analysis, and interpretation of data. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Disclosure
There are no conflicts of interest.
Ethical Approval
The research contents and procedures have been approved by the Institutional Review Committee of the Sixth Affiliated Hospital of Sun Yat sen University (Project's number: E2021156). In this study, all patient information was treated anonymously and confidentially, so informed consent was waived.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, M., Deng, S., Cao, Y. et al. Mitochondrial DNA Copy Number as a Biomarker for Guiding Adjuvant Chemotherapy in Stages II and III Colorectal Cancer Patients with Mismatch Repair Deficiency: Seeking Benefits and Avoiding Harms. Ann Surg Oncol (2024). https://doi.org/10.1245/s10434-024-15759-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1245/s10434-024-15759-y