Abstract
Background
D-dimer (DDI) and albumin are prognostic markers for numerous cancers; however, the predictive value of the preoperative DDI-to-albumin ratio (DAR) on the survival and recurrence patterns of gastric cancer (GC) remains unclear.
Objective
The aim of this study was to explore the prognostic value of the DAR in GC.
Methods
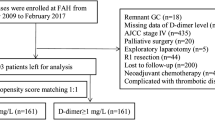
Our study included 1766 patients with GC, divided into training and testing cohorts at a ratio of 7:3. Patients were classified into either a high-DAR group (> 0.0145) or low-DAR group (≤ 0.0145) according to the cut-off value of receiver operating characteristic (ROC) curve analysis. The relationship between the DAR and recurrence pattern was analyzed in stage II/III patients.
Results
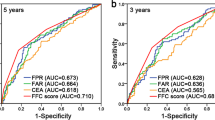
Eight preoperative hematological factors were included and 17 composite inflammatory markers were constructed. ROC and random forest analyses indicated that among 17 markers, DAR was the best predictor for overall survival (OS) in GC (p < 0.01). High DAR was significantly associated with poor OS (hazard ratio [HR] 1.89, p < 0.001) and recurrence-free survival (RFS; HR 1.85, p < 0.001). Subgroup analysis showed no differences in OS and RFS between the high- and low-DAR groups in stage I or pT1/2 or pN0/1 patients; however, in stage II/III or pT3/4 or pN2/3 patients, the high-DAR group had shorter OS and RFS rates than the low-DAR group (p < 0.001). Similar results were found in the testing cohort. According to the multivariate analysis based on the training cohort, five indices, including DAR, cT stage, cN stage, age and body mass index (BMI), were incorporated to establish a nomogram model to predict the long-term prognosis of GC. The model showed comparable forecast performance in predicting OS (C-index: 0.773 vs. 0.786) and RFS (C-index: 0.788 vs. 0.795) compared with pTNM. Recurrence pattern analysis in stage II/III patients showed that the high-DAR group had a higher incidence of peritoneal implantation and early recurrence (ER) than the low-DAR group, and the post-recurrence survival in the high-DAR group was significantly shorter than that in the low-DAR group (p = 0.016).
Conclusion
The preoperative DAR is a new biomarker for the long-term survival prediction of GC. In advanced GC, a preoperative DAR > 0.0145 aids the timely detection of ER and peritoneal recurrence after surgery, thus guiding individual follow-up strategies.






Similar content being viewed by others
References
Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. Int J Mol Sci. 2020;21(11):4012. https://doi.org/10.3390/ijms21114012.
Ay C, Dunkler D, Pirker R, et al. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica. 2012;97(8):1158–64. https://doi.org/10.3324/haematol.2011.054718.
Cupp MA, Cariolou M, Tzoulaki I, Aune D, Evangelou E, Berlanga-Taylor AJ. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020;18(1):360. https://doi.org/10.1186/s12916-020-01817-1.
Saito R, Shoda K, Maruyama S, et al. Platelets enhance malignant behaviours of gastric cancer cells via direct contacts. Br J Cancer. 2021;124(3):570–3. https://doi.org/10.1038/s41416-020-01134-7.
Lu J, Xu BB, Zheng ZF, et al. CRP/prealbumin, a novel inflammatory index for predicting recurrence after radical resection in gastric cancer patients: post hoc analysis of a randomized phase III trial. Gastric Cancer. 2019;22(3):536–45. https://doi.org/10.1007/s10120-018-0892-0.
Pang H, Zhang W, Liang X, et al. Prognostic score system using preoperative inflammatory, nutritional and tumor markers to predict prognosis for gastric cancer: a two-center cohort study. Adv Ther. 2021;38(9):4917–34. https://doi.org/10.1007/s12325-021-01870-z.
Lin Y, Liu Z, Qiu Y, et al. Clinical signifificance of plasma D-dimer and fifibrinogen in digestive cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2018;44(10):1494–503. https://doi.org/10.1016/j.ejso.2018.07.052.
Kawai K, Watanabe T. Colorectal cancer and hypercoagulability. Surg Today. 2014;44(5):797–803. https://doi.org/10.1007/s00595-013-0606-5.
Hu C, Chen R, Chen W, et al. Thrombocytosis is a significant indictor of hypercoagulability, prognosis and recurrence in gastric cancer. Exp Ther Med. 2014;8(1):125–32. https://doi.org/10.3892/etm.2014.1699.
Blackwell K, Haroon Z, Broadwater G, et al. Plasma D-dimer levels in operable breast cancer patients correlate with clinical stage and axillary lymph node status. J Clin Oncol. 2000;18(3):600–8. https://doi.org/10.1200/JCO.2000.18.3.600.
Blackwell K, Hurwitz H, Liebérman G, et al. Circulating D-dimer levels are better predictors of overall survival and disease progression than carcinoembryonic antigen levels in patients with metastatic colorectal carcinoma. Cancer. 2004;101(1):77–82. https://doi.org/10.1002/cncr.20336.
Xu BB, Lu J, Zheng ZF, et al. The predictive value of the preoperative C-reactive protein-albumin ratio for early recurrence and chemotherapy benefit in patients with gastric cancer after radical gastrectomy: using randomized phase III trial data. Gastric Cancer. 2019;22(5):1016–28. https://doi.org/10.1007/s10120-019-00936-w.
Lin GS, Huang XY, Lu J, et al. A good preoperative immune prognostic index is predictive of better long-term outcomes after laparoscopic gastrectomy compared with open gastrectomy for stage II gastric cancer in elderly patients. Surg Endosc. 2022;36(3):1814–26. https://doi.org/10.1007/s00464-021-08461-7.
Lin GT, Ma YB, Chen QY, et al. Fibrinogen-albumin ratio as a new promising preoperative biochemical marker for predicting oncological outcomes in gastric cancer: a multi-institutional study. Ann Surg Oncol. 2021;28(12):7063–73. https://doi.org/10.1245/s10434-021-10027-9.
Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual. 8th edn. New York: Springer; 2016.
Iwasaki Y, Terashima M, Mizusawa J, et al. Gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer (JCOG0501): an open-label, phase 3, randomized controlled trial. Gastric Cancer. 2021;24(2):492–502. https://doi.org/10.1007/s10120-020-01136-7.
Hu Y, Huang C, Sun Y, et al. Morbidity and mortality of laparoscopic versus open D2 distal gastrectomy for advanced gastric cancer: a randomized controlled trial. J Clin Oncol. 2016;34(12):1350–7. https://doi.org/10.1200/JCO.2015.63.7215.
Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–21. https://doi.org/10.1016/S0140-6736(11)61873-4.
Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. https://doi.org/10.1056/NEJMoa055531.
Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29(33):4387–93. https://doi.org/10.1200/JCO.2011.36.5908.
Zheng H, Zhao Y, He Q, et al. Multi-institutional development and validation of a nomogram to predict recurrence after curative resection of gastric neuroendocrine/mixed adenoneuroendocrine carcinoma. Gastric Cancer. 2021;24(2):503–14.
Jiao X, Wang Y, Wang F, Wang X. Recurrence pattern and its predictors for advanced gastric cancer after total gastrectomy. Medicine. 2020;99(51):e23795. https://doi.org/10.1097/MD.0000000000023795.
Lu J, Xu BB, Zheng CH, et al. Development and external validation of a nomogram to predict recurrence-free survival after R0 resection for stage II/III gastric cancer: an international multicenter study. Front Oncol. 2020;10:574611. https://doi.org/10.3389/fonc.2020.574611.
Piazuelo MB, Riechelmann RP, Wilson KT, Algood HMS. Resolution of gastric cancer-promoting inflammation: a novel strategy for anti-cancer therapy. Curr Top Microbiol Immunol. 2019;421:319–59. https://doi.org/10.1007/978-3-030-15138-6_13.
Kanda M, Tanaka C, Kobayashi D, et al. Proposal of the coagulation score as a predictor for short-term and long-term outcomes of patients with resectable gastric cancer. Ann Surg Oncol. 2017;24(2):502–9. https://doi.org/10.1245/s10434-016-5544-1.
He SS, Wang Y, Yang L, et al. Plasma fibrinogen correlates with metastasis and is associated with prognosis in human nasopharyngeal carcinoma. J Cancer. 2017;8(3):403–9. https://doi.org/10.7150/jca.17028.
Lin Y, Liu Z, Qiu Y, et al. Clinical significance of plasma D-dimer and fibrinogen in digestive cancer: a systematic review and meta-analysis. Eur J Surg Oncol. 2018;44(10):1494–503. https://doi.org/10.1016/j.ejso.2018.07.052.
Batschauer APB, Figueiredo CP, Bueno EC, et al. D-dimer as a possible prognostic marker of operable hormone receptor-negative breast cancer. Ann Oncol. 2010;21(6):1267–72. https://doi.org/10.1093/annonc/mdp474.
Dai H, Zhou H, Sun Y, et al. D-dimer as a potential clinical marker for predicting metastasis and progression in cancer. Biomed Rep. 2018;9(5):453–7. https://doi.org/10.3892/br.2018.1151.
Di Nisio M, Klerk CP, Meijers JC, Büller HR. The prognostic value of the D-dimer test in cancer patients treated with and without low-molecular-weight heparin. J Thromb Haemost. 2005;3(7):1531–3. https://doi.org/10.1111/j.1538-7836.2005.01413.x.
Liu DQ, Li FF, Jia WH. Cumulative scores based on plasma D-dimer and serum albumin levels predict survival in esophageal squamous cell carcinoma patients treated with transthoracic esophagectomy. Chin J Cancer. 2016;35:11. https://doi.org/10.1186/s40880-015-0062-2.
Desch A, Gebhardt C, Utikal J, Schneider SW. D-dimers in malignant melanoma: association with prognosis and dynamic variation in disease progress. Int J Cancer. 2017;140(4):914–21. https://doi.org/10.1002/ijc.30498.
Edwards CM, Warren J, Armstrong L, Donnelly PK. D-dimer: a useful marker of disease stage in surgery for colorectal cancer. Br J Surg. 1993;80(11):1404–5. https://doi.org/10.1002/bjs.1800801116.
Kilic L, Yildiz I, Sen FK, et al. D-dimer and international normalized ratio (INR) are correlated with tumor markers and disease stage in colorectal cancer patients. Cancer Biomark. 2015;15(4):405–11. https://doi.org/10.3233/CBM-150477.
Wiedermann CJ. Hypoalbuminemia as surrogate and culprit of infections. Int J Mol Sci. 2021;22(9):4496. https://doi.org/10.3390/ijms22094496.
Barreiro E. Models of disuse muscle atrophy: therapeutic implications in critically ill patients. Ann Transl Med. 2018;6(2):29. https://doi.org/10.21037/atm.2017.12.12.
Haskins IN, Baginsky M, Amdur RL, Agarwal S. Preoperative hypoalbuminemia is associated with worse outcomes in colon cancer patients. Clin Nutr. 2017;36(5):1333–8. https://doi.org/10.1016/j.clnu.2016.08.023.
Morhij R, Mahendra A, Jane M, McMillan DC. The modified Glasgow prognostic score in patients undergoing surgery for bone and soft tissue sarcoma. J Plast Reconstr Aesthet Surg. 2017;70(5):618–24. https://doi.org/10.1016/j.bjps.2017.01.016.
Lin JX, Wang ZK, Huang YQ, et al. Dynamic changes in pre- and postoperative levels of inflammatory markers and their effects on the prognosis of patients with gastric cancer. J Gastrointest Surg. 2021;25(2):387–96. https://doi.org/10.1007/s11605-020-04523-8.
Wang Q, Tan X, Deng G, Fu S, Li J, Li Z. Dynamic changes in the systemic immune-inflammation index predict the prognosis of EGFR-mutant lung adenocarcinoma patients receiving brain metastasis radiotherapy. BMC Pulm Med. 2022;22(1):75. https://doi.org/10.1186/s12890-022-01866-7.
Zhou ZQ, Pang S, Yu XC, et al. Predictive values of postoperative and dynamic changes of inflammation indexes in survival of patients with resected colorectal cancer. Curr Med Sci. 2018;38(5):798–808. https://doi.org/10.1007/s11596-018-1946-6.
Zhang L, Wang Z, **ao J, Zhang Z, Li H, Li F, Zhang L, Wang Y. Prognostic value of albumin to D-dimer ratio in advanced gastric cancer. J Oncol. 2021;2021:9973743. https://doi.org/10.1155/2021/9973743.
Ren B, Cui M, Yang G, et al. Tumor microenvironment participates in metastasis of pancreatic cancer. Mol Cancer. 2018;17(1):108. https://doi.org/10.1186/s12943-018-0858-1.
Oya M, Akiyama Y, Yanagida T, Akao S, Ishikawa H. Plasma D-dimer level in patients with colorectal cancer: its role as a tumor marker. Surg Today. 1998;28(4):373–8. https://doi.org/10.1007/s005950050144.
Lu Z, Fang Y, Liu C, et al. Early interdisciplinary supportive care in patients with previously untreated metastatic esophagogastric cancer: a phase III randomized controlled trial. J Clin Oncol. 2021;39(7):748–56. https://doi.org/10.1200/JCO.20.01254.
Acknowledgements
The authors are grateful to the Chinese Gastric Cancer Association, the Chinese Society of Laparo-Endoscopic Surgery, and the Chinese Society of Gastrointestinal Surgery for their scientific support.
Author information
Authors and Affiliations
Contributions
Guosheng Lin and Jun Lu contributed to the conception and design of the study. Dong Wu, Bin-Bin Xu, Zhen Xue, Jia Lin, Lili Shen, and Hualong Zheng contributed to the follow-up and acquisition, analysis and interpretation of data. Guosheng Lin and Jun Lu participated in drafting the article. ** Li, Jian-Wei **e and Qi-Yue Chen participated in revising the article critically for important intellectual content. Chang-Ming Huang and Chao-Hui Zheng provided final approval of the version to be published.
Corresponding author
Ethics declarations
Disclosure
Guo-Sheng Lin, Jun Lu, Jia Lin, Hua-Long Zheng, Bin-Bin Xu, Zhen Xue, Dong Wu, Lili Shen, Chao-Hui Zheng, **-Li, Jian-Wei **e, Qi-Yue Chen, and Chang-Ming Huang have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, GS., Lu, J., Lin, J. et al. Value of the Preoperative D-Dimer to Albumin Ratio for Survival and Recurrence Patterns in Gastric Cancer. Ann Surg Oncol 30, 1132–1144 (2023). https://doi.org/10.1245/s10434-022-12625-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12625-7