Abstract
Background
The optimal lymph node (LN) dissection for left-sided pancreatic cancer based on tumor location has remained unknown. In particular, the efficacy of LN dissection around the common hepatic artery and the celiac axis for distal tumors has not been established. This study was designed to elucidate the frequency and prognostic impact of LN metastasis, focusing on tumor location.
Methods
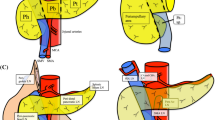
Data from 110 patients with invasive pancreatic cancer who underwent distal pancreatectomy between 2007 and 2020 were collected. We used a quantitative value―the distance between the left side of the portal vein and the right side of tumor (DPT)―to define the tumor location. LN stations were divided into two groups: peripancreatic lymph nodes (PLN) and non-PLN. We then analyzed the frequency of LN metastasis based on the tumor location and prognostic factors.
Results
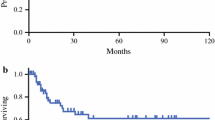
Non-PLN metastasis was observed in 7.3% of patients. Non-PLN metastasis was found only in patients with a DPT < 20 mm. Patients with non-PLN metastasis exhibited a significantly worse prognosis than those with only-PLN metastasis (median survival time: 20.3 vs. 42.5 months, p = 0.048). Multivariate analysis for survival indicated that tumor size > 4 cm (hazard ratio [HR]: 2.23, p = 0.012) and metastasis in the non-PLN region (HR: 3.02, p = 0.015), and inability to undergo adjuvant chemotherapy (HR: 2.81, p = 0.0018) were also associated with poor prognosis.
Conclusions
Dissection of the non-PLN region can be avoided in selected patients with DPT ≥ 20 mm.



Similar content being viewed by others
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. https://doi.org/10.3322/caac.21654.
Yeo CJ, Cameron JL, Sohn TA, et al. Pancreaticoduodenectomy with or without extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma: comparison of morbidity and mortality and short-term outcome. Ann Surg. 1999;229(5):613–24. https://doi.org/10.1097/00000658-199905000-00003.
Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236(3):355.
Farnell MB, Pearson RK, Sarr MG, et al. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery. 2005;138(4):618–30.
Nimura Y, Nagino M, Takao S, et al. Standard versus extended lymphadenectomy in radical pancreatoduodenectomy for ductal adenocarcinoma of the head of the pancreas. J Hepatobiliary Pancreat Sci. 2012;19(3):230–41.
Jang J-Y, Kang MJ, Heo JS, et al. A prospective randomized controlled study comparing outcomes of standard resection and extended resection, including dissection of the nerve plexus and various lymph nodes, in patients with pancreatic head cancer. Ann Surg. 2014;259(4):656–64.
Jang J, Kang JS, Han Y, et al. Long-term outcomes and recurrence patterns of standard versus extended pancreatectomy for pancreatic head cancer: a multicenter prospective randomized controlled study. J Hepato-biliary-pancreatic Sci. 2017;24(7):426–33.
Japan Pancreas Society. Classification of pancreatic carcinoma. In: The Japan Pancreas Society: Classification of Pancreatic Carcinoma; 2017.
Tol JAMG, Gouma DJ, Bassi C, et al. Definition of a standard lymphadenectomy in surgery for pancreatic ductal adenocarcinoma: a consensus statement by the International Study Group on Pancreatic Surgery (ISGPS). Surg (United States). 2014;156(3):591–600. https://doi.org/10.1016/j.surg.2014.06.016.
Nakao A, Harada A, Nonami T, et al. Lymph node metastasis in carcinoma of the body and tail of the pancreas. Br J Surg. 1997;84(8):1090–2. https://doi.org/10.1002/bjs.1800840813.
Fujita T, Nakagohri T, Gotohda N, et al. Evaluation of the prognostic pactors and significance of lymph node status in invasive ductal carcinoma of the body or tail of the pancreas. Pancreas. 2010;39(1):48–54. https://doi.org/10.1097/MPA.0b013e3181bd5cfa.
Sahin TT, Fujii T, Kanda M, et al. Prognostic implications of lymph node metastases in carcinoma of the body and tail of the pancreas. Pancreas. 2011;40(7):1029–33. https://doi.org/10.1097/MPA.0b013e3182207893.
Kanda M, Fujii T, Nagai S, et al. Pattern of lymph node metastasis spread in pancreatic cancer. Pancreas. 2011;40(6):951–5. https://doi.org/10.1097/MPA.0b013e3182148342.
Tanaka K, Nakamura T, Asano T, et al. Pancreatic body and tail cancer and favorable metastatic lymph node behavior on the left edge of the aorta. Pancreatology. 2020;20(7):1451–7. https://doi.org/10.1016/j.pan.2020.08.014.
Imamura T, Yamamoto Y, Sugiura T, et al. Reconsidering the optimal regional lymph node station according to tumor location for pancreatic cancer. Ann Surg Oncol. 2021;28(3):1602–11. https://doi.org/10.1245/s10434-020-09066-5.
Yamada S, Takeda S, Fujii T, et al. Clinical implications of peritoneal cytology in potentially resectable pancreatic cancer: positive peritoneal cytology may not confer an adverse prognosis. Ann Surg. 2007;246(2):254–8. https://doi.org/10.1097/01.sla.0000261596.43439.92.
Yoshioka R, Saiura A, Koga R, et al. The implications of positive peritoneal lavage cytology in potentially resectable pancreatic cancer. World J Surg. 2012;36(9):2187–91. https://doi.org/10.1007/s00268-012-1622-0.
Yamada S, Fujii T, Kanda M, et al. peritoneal cytology in potentially resectable pancreatic cancer. Br J Surg. 2013;100(13):1791–6.
Strasberg SM, Linehan DC, Hawkins WG. Radical antegrade modular pancreatosplenectomy procedure for adenocarcinoma of the body and tail of the pancreas: ability to obtain negative tangential margins. J Am Coll Surg. 2007;204(2):244–9.
Strasberg SM, Drebin JA, Linehan D. Radical antegrade modular pancreatosplenectomy. Surgery. 2003;133(5):521–7.
Unno M, Motoi F, Matsuyama Y, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP-05). 2019.
Ielpo B, Duran H, Diaz E, et al. Preoperative treatment with gemcitabine plus nab-paclitaxel is a safe and effective chemotherapy for pancreatic adenocarcinoma. Eur J Surg Oncol. 2016;42(9):1394–400.
Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817–25.
Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: The CONKO-001 randomized trial. JAMA—J Am Med Assoc. 2013;310(14):1473–81. https://doi.org/10.1001/jama.2013.279201.
Uesaka K, Boku N, Fukutomi A, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016;388(10041):248–57. https://doi.org/10.1016/S0140-6736(16)30583-9.
Brierley JD, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumours. New York: Wiley; 2017.
Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48(3):452–8. https://doi.org/10.1038/bmt.2012.244.
Strobel O, Hinz U, Gluth A, et al. Pancreatic adenocarcinoma: number of positive nodes allows to distinguish several N categories. Ann Surg. 2015;261(5):961–9. https://doi.org/10.1097/SLA.0000000000000814.
Warschkow R, Tsai C, Köhn N, et al. Role of lymphadenectomy, adjuvant chemotherapy, and treatment at high-volume centers in patients with resected pancreatic cancer—a distinct view on lymph node yield. Langenbeck’s Arch Surg. 2020;405(1):43–54. https://doi.org/10.1007/s00423-020-01859-2.
Malleo G, Maggino L, Nobile S, et al. Reappraisal of nodal staging and study of lymph node station involvement in distal pancreatectomy for body-tail pancreatic ductal adenocarcinoma. Eur J Surg Oncol. 2020;46(9):1734–41. https://doi.org/10.1016/j.ejso.2020.04.006.
Sasako M, McCulloch P, Kinoshita T, Maruyama K. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg. 1995;82(3):346–51. https://doi.org/10.1002/bjs.1800820321.
Zhou Y, Lin J, Wang W, et al. Should a standard lymphadenectomy include the No. 9 lymph nodes for body and tail pancreatic ductal adenocarcinoma? Pancreatology. 2019;19(3):414–48. https://doi.org/10.1016/j.pan.2019.03.005.
Acknowledgments
The authors thank Editage (www.editage.com) for English language editing.
Funding
The authors affirm that they have no financial or personal affiliations or other involvement with any commercial organization that has a direct financial interest in any matter included in this manuscript.
Author information
Authors and Affiliations
Contributions
Conception and design of the work: HI, TO; Data analysis and interpretation: HI, TO, AT, RM, SM, KA; Writing—original draft: HI; Writing—review and editing: TO, AT, KA; Supervision: MT, YK; All authors reviewed the results and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Disclosure
The authors declare that they have no conflicts of interest that are directly relevant to the content of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ishida, H., Ogura, T., Takahashi, A. et al. Optimal Region of Lymph Node Dissection in Distal Pancreatectomy for Left-Sided Pancreatic Cancer Based on Tumor Location. Ann Surg Oncol 29, 2414–2424 (2022). https://doi.org/10.1245/s10434-021-11108-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-11108-5