Abstract
Introduction
Studies have shown that stem cells exert their therapeutic effects on acute kidney injury (AKI) through paracrine/endocrine actions. If the protective effect is mediated in an endocrine manner, the injection of the factors that these cells secrete could be effective, but the effect of conditioned medium (CM) remains controversial.
Methods
In this study, we cultured mesenchymal stem cells (MSCs) and then transplanted them into an ischemia-reperfusion (I/R) injury model. CM was also injected into mice, and the histological changes, level of cell proliferation, loss of peritubular capillaries and anti-inflammatory and anti-apoptotic effects were examined at different time points.
Results
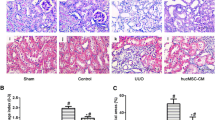
The results showed that MSC infusion improved renal function and histological alterations, leading to significantly reduced mortality. MSC administration also promoted kidney microvasculature repair, attenuated kidney peritubular capillary loss, increased the proliferation of parenchymal cells and decreased CD68-positive macrophage infiltration and apoptotic cells. Although we determined that CM contained proangiogenic factors, including hepatocyte growth factor (HGF), vascular endothelial growth factor-A (VEGF-A) and insulin-like growth factor-1 (IGF-1), no favorable effects were observed during the course of repair.
Conclusions
Our data show that MSC infusion promotes kidney repair in a variety of ways, including enhancement of the repair of peritubular capillaries and tubular epithelial cells and anti-inflammatory and anti-apoptotic effects. MSCs can secrete high levels of proangiogenic growth factors, but CM results in a nonsignificant improvement, indicating that MSCs play a role in kidney repair through paracrine rather than endocrine mechanisms. These results indicate that MSC infusion is a promising therapeutic strategy for promoting kidney repair after injury.
Similar content being viewed by others
Introduction
Acute kidney injury (AKI) is one of the most important causes of mortality and morbidity worldwide. In clinical practice, kidney ischemia–reperfusion (I/R) is the most common cause of AKI. Limitations in the treatment have led to a search for better therapeutic options. Mesenchymal stem cell (MSC)-based therapy holds great promise for treating immune disorders and for regenerative medicine, and promising results have been reported for the application of different types of stem cells in the treatment of kidney failure [1–5, 19–21]. In addition, emerging evidence indicates that AKI in humans is closely associated with chronic kidney disease if the repair process is maladaptive [22, 23]. However, the therapeutic options are limited.
Bone marrow stem cells are an attractive therapy to promote renal tissue regeneration due to their pluripotency and ease of isolation. Using these cells also avoids the ethical ambiguities of using embryonic stem cells [4, 15, 24, 25]. Our previous studies also demonstrated that hematopoietic stem cells recruited to injured kidneys generate high levels of proangiogenic cytokines, including VEGF-A [8]. This result increased our interest in determining whether CM had beneficial effects on kidney repair.
In the present study, we obtained MSCs using typical methods and cultured these cells for four passages before use in our experiments. Light microscopy showed that these cells had typical spindle-shaped morphology and were well labeled with CMFDA. Additionally, we demonstrated that MSCs that were systemically infused 24 hours after kidney injury were selectively recruited to injured kidneys. This recruitment was associated with enhanced repair of the microvasculature and tubules, improved kidney function, increased survival, promoted the proliferation of parenchymal cells, and decreased CD68-positive macrophage infiltration and apoptotic cells. In contrast, systemic CM treatment did not have any significantly beneficial effects, even though the CM contained high levels of proangiogenic cytokines, including HGF, VEGF-A and IGF-1.
Acute ischemic injury in the kidneys primarily results in proximal tubular damage [6, 26, 27]. However, data derived from several severe AKI models and the long-term effects of ischemic injury demonstrate that capillary loss typically precedes the development of prominent renal fibrosis, the loss of capillary density and blood flow may result in poor delivery of oxygen and nutrients to the damaged area, and neoangiogenesis may be a central process in the preservation of the vascular structure and the restoration of organ function [28–31]. In this study, we demonstrated that there was a marked loss of peritubular capillaries in the injured kidneys, and that the intravenous infusion of MSCs attenuated the loss of peritubular capillaries and tubular injury and promoted cell proliferation in the kidney. These effects were associated with both the rapid recovery of kidney function and the enhanced survival of the mice.
The critical property of stem cells is that they are able to generate many or all differentiated cell types [32, 33]. Initial studies reported that bone-marrow derived stem cells can differentiate into endothelial and mesangial cells in animal models [34–36], but the number of differentiated cells was small. Recently, it was found that MSCs can produce many growth factors, suggesting that a paracrine/endocrine effect might contribute to renal protection [2, 4, 12]. Gharaibeh and colleagues have shown that the terminal differentiation capacity of implanted stem cells is not the major determinant of the cells’ regenerative potential and that the paracrine effect imparted by the transplanted cells plays a greater role in the regeneration process [37]. Zarjou and colleagues have further shown that heme oxygenase-1 enhances secretion of stromal cell-derived factor-1, VEGF-A and HGF by MSCs [38]. Many findings support a protective effect mediated in an endocrine manner, which, if true, would mean that injection of the cells themselves would not be required, and the factors that these cells secrete could be effective. The effect of CM, however, remains controversial for the moment [12, 39]. In this study we also determined the levels of HGF, VEGF-A and IGF-1, and the data showed that CM contained these factors, which have renoprotective effects after AKI. Based on these results, we hypothesized that administering the CM would protect against kidney failure, making it unnecessary to transplant stem cells and thus avoiding the risks of tumorigenesis and immunologic reactions. However, we did not observe any favorable effects in the CM group on renal function, histological alterations or cell proliferation and anti-inflammatory and anti-apoptotic effects, even though we increased the dose and repeated consecutive administration of CM. There are several possible explanations for these findings. First, the AKI injury models were induced by different methods, and we believe that the outcomes should be compared within a unique and identical model and cannot be meaningfully transposed from one model to another. Second, the microenvironment has very important effects on the production of growth factors by MSCs. Different microenvironments can stimulate stem cells to release different types and concentrations of cytokines. MSCs might secrete another set of mediators in the culture system [12]. If we want stem cells to have the same effects in vitro and in vivo, we must mimic the injury microenvironment in the culture system. In the I/R model, the loss of blood flow results in hypoxia in the tissue, and the bone marrow is also hypoxic [40, 41]. We therefore believe that the MSCs should be exposed to hypoxic conditions to mimic the in vivo environment. Some authors have performed these types of experiments [42–44]. Third, the timing of therapeutic cell delivery may be critical. Cellular populations within wounds change depending on the phases of the repair process. This change means that therapeutic cells will encounter different microenvironments at each stage of the repair process [45].
In contrast with our data, Bi and colleagues reported that administration of MSC CM was very potent in ameliorating cisplatin-induced kidney failure [12]. Comparing these two studies, there are some differences. First, the medium was harvested after 96 hours as CM but in our study was harvested after 48 hours. Second, Bi and colleagues infused 1000 μl CM twice per day for 6 days by intraperitoneal injection, and we injected 200 μl or 500 μl CM intravenously through the tail vein once per day for 7 days. Third, they gave an intraperitoneal injection of cisplatin to induce acute tubular injury, but we placed a nontraumatic microaneurysm clamp across the renal artery and vein to induce kidney I/R injury. Fourth, different mouse strains were used in these two studies (C57BI/6 compared with BALB/C). We consider that these differences account for the discrepancies in the findings at least in part. We believe the that therapeutic strategy for treatment of kidney disease with CM remains an open question, and further studies with different designs, animal models and evaluation methods are certainly required.
Conclusions
We demonstrate that systematically administered MSCs promote rapid kidney repair and reduce mortality. Our data supporting the fact that the beneficial effect seen with MSCs is probably due to the stem cells’ multipotent capacity include increased secretion of paracrine factors, improved angiogenic and anti-inflammatory activities and anti-apoptotic effects. The results of this study indicate that the MSC infusion is a promising therapeutic strategy for AKI. In the present study, we do not detect any beneficial role of CM in our animal model, indicating that MSCs play central roles in kidney repair through paracrine rather than endocrine mechanisms. We believe that considerable work with different designs and animals is still required.
Abbreviations
- AKI:
-
acute kidney injury
- BUN:
-
blood urea nitrogen
- CM:
-
conditioned medium
- CMFDA:
-
5-chloromethylfluorescein diacetate
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- HGF:
-
hepatocyte growth factor
- I/R:
-
ischemia–reperfusion
- IGF-1:
-
insulin-like growth factor-1
- MSC:
-
mesenchymal stem cell
- PBS:
-
phosphate-buffered saline
- TUNEL:
-
terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling
- VEGF-A:
-
vascular endothelial growth factor-A.
References
Morigi M, Introna M, Imberti B, Corna D, Abbate M, Rota C, Rottoli D, Benigni A, Perico N, Zoja C: Human bone marrow mesenchymal stem cells accelerate recovery of acute renal injury and prolong survival in mice. Stem Cells. 2008, 26: 2075-2082. 10.1634/stemcells.2007-0795.
Tögel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C: Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007, 292: F1626-F1635. 10.1152/ajprenal.00339.2006.
Prodromidi EI, Poulsom R, Jeffery R, Roufosse CA, Pollard PJ, Pusey CD, Cook HT: Bone marrow-derived cells contribute to podocyte regeneration and amelioration of renal disease in a mouse model of Alport syndrome. Stem Cells. 2006, 24: 2448-2455. 10.1634/stemcells.2006-0201.
Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C: Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005, 289: F31-F42. 10.1152/ajprenal.00007.2005.
Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, Rottoli D, Angioletti S, Benigni A, Perico N: Mesenchymal stem cells are renotropic, hel** to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004, 15: 1794-1804. 10.1097/01.ASN.0000128974.07460.34.
Lin F, Cordes K, Li L, Hood L, Couser WG, Shankland SJ, Igarashi P: Hematopoietic stem cells contribute to the regeneration of renal tubules after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2003, 14: 1188-1199. 10.1097/01.ASN.0000061595.28546.A0.
Eliopoulos N, Zhao J, Forner K, Birman E, Young YK, Bouchentouf M: Erythropoietin gene-enhanced marrow mesenchymal stromal cells decrease cisplatin-induced kidney injury and improve survival of allogeneic mice. Mol Ther. 2011, 19: 2072-2083. 10.1038/mt.2011.162.
Li B, Cohen A, Hudson TE, Motlagh D, Amrani DL, Duffield JS: Mobilized human hematopoietic stem/progenitor cells promote kidney repair after ischemia/reperfusion injury. Circulation. 2010, 121: 2211-2220. 10.1161/CIRCULATIONAHA.109.928796.
Kunter U, Rong S, Boor P, Eitner F, Müller-Newen G, Djuric Z, van Roeyen CR, Konieczny A, Ostendorf T, Villa L: Mesenchymal stem cells prevent progressive experimental renal failure but maldifferentiate into glomerular adipocytes. J Am Soc Nephrol. 2007, 18: 1754-1764. 10.1681/ASN.2007010044.
Du T, Zou X, Cheng J, Wu S, Zhong L, Ju G, Zhu J, Liu G, Zhu Y, **a S: Human Wharton's jelly-derived mesenchymal stromal cells reduce renal fibrosis through induction of native and foreign hepatocyte growth factor synthesis in injured tubular epithelial cells. Stem Cell Res Ther. 2013, 4: 59-10.1186/scrt215.
Reis LA, Borges FT, Simões MJ, Borges AA, Sinigaglia-Coimbra R, Schor N: Bone marrow-derived mesenchymal stem cells repaired but did not prevent gentamicin-induced acute kidney injury through paracrine effects in rats. PLoS One. 2012, 7: e44092-10.1371/journal.pone.0044092.
Bi B, Schmitt R, Israilova M, Nishio H, Cantley LG: Stromal cells protect against acute tubular injury via an endocrine effect. J Am Soc Nephrol. 2007, 18: 2486-2496. 10.1681/ASN.2007020140.
Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Falda M, Bussolati B, Tetta C: Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009, 20: 1053-1067. 10.1681/ASN.2008070798.
Liew A, O’Brien T: Therapeutic potential for mesenchymal stem cell transplantation in critical limb ischemia. Stem Cell Res Ther. 2012, 3: 28-10.1186/scrt119.
Humphreys BD, Bonventre JV: Mesenchymal stem cells in acute kidney injury. Annu Rev Med. 2008, 59: 311-325. 10.1146/annurev.med.59.061506.154239.
Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L: Mesenchymal stem cell–natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006, 107: 1484-1490. 10.1182/blood-2005-07-2775.
Duffield JS, Park KM, Hsiao L, Kelley VR, Scadden DT, Ichimura T, Bonventre JV: Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest. 2005, 115: 1743-10.1172/JCI22593.
Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ: Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004, 103: 1662-1668. 10.1182/blood-2003-09-3070.
Cantaluppi V, Biancone L, Romanazzi GM, Figliolini F, Beltramo S, Galimi F, Camboni MG, Deriu E, Conaldi P, Bottelli A: Macrophage stimulating protein may promote tubular regeneration after acute injury. J Am Soc Nephrol. 2008, 19: 1904-1918. 10.1681/ASN.2007111209.
Nash K, Hafeez A, Hou S: Hospital-acquired renal insufficiency. Am J Kidney Dis. 2002, 39: 930-936. 10.1053/ajkd.2002.32766.
Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, Paganini EP, Chertow GM: Spectrum of acute renal failure in the intensive care unit: the PICARD experience. Kidney Int. 2004, 66: 1613-1621. 10.1111/j.1523-1755.2004.00927.x.
Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, Collins AJ: Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol. 2009, 20: 223-228. 10.1681/ASN.2007080837.
Bagshaw SM, George C, Bellomo R: A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008, 23: 1569-1574. 10.1093/ndt/gfn009.
Roufosse C, Cook H: Stem cells and renal regeneration. Nephron Exp Nephrol. 2008, 109: e39-e45. 10.1159/000139989.
Yeagy BA, Cherqui S: Kidney repair and stem cells: a complex and controversial process. Pediatr Nephrol. 2011, 26: 1427-1434. 10.1007/s00467-011-1789-x.
Vogetseder A, Palan T, Bacic D, Kaissling B, Le Hir M: Proximal tubular epithelial cells are generated by division of differentiated cells in the healthy kidney. Am J Physiol Cell Physiol. 2007, 292: C807-C813.
Basile DP, Donohoe D, Roethe K, Osborn JL: Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001, 281: F887-F899.
Basile D: The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int. 2007, 72: 151-156. 10.1038/sj.ki.5002312.
Basile DP: Challenges of targeting vascular stability in acute kidney injury. Kidney Int. 2008, 74: 257-258. 10.1038/ki.2008.243.
Reinders MEJ, Rabelink TJ, Briscoe DM: Angiogenesis and endothelial cell repair in renal disease and allograft rejection. J Am Soc Nephrol. 2006, 17: 932-942. 10.1681/ASN.2005121250.
Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ: Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol. 2001, 12: 1448-1457.
Romagnani P: Toward the identification of a ‘renopoietic system’?. Stem Cells. 2009, 27: 2247-2253. 10.1002/stem.140.
Blau H, Brazelton T, Weimann J: The evolving concept review of a stem cell: entity or function?. Cell. 2001, 105: 829-841. 10.1016/S0092-8674(01)00409-3.
Masuya M, Drake CJ, Fleming PA, Reilly CM, Zeng H, Hill WD, Martin-Studdard A, Hess DC, Ogawa M: Hematopoietic origin of glomerular mesangial cells. Blood. 2003, 101: 2215-2218. 10.1182/blood-2002-04-1076.
Ito T, Suzuki A, Imai E, Okabe M, Hori M: Bone marrow is a reservoir of repopulating mesangial cells during glomerular remodeling. J Am Soc Nephrol. 2001, 12: 2625-2635.
Rookmaaker MB, Smits AM, Tolboom H, van't Wout K, Martens AC, Goldschmeding R, Joles JA, Van Zonneveld AJ, Gröne HJ, Rabelink TJ: Bone-marrow-derived cells contribute to glomerular endothelial repair in experimental glomerulonephritis. Am J Pathol. 2003, 163: 553-562. 10.1016/S0002-9440(10)63683-8.
Gharaibeh B, Lavasani M, Cummins JH, Huard J: Terminal differentiation is not a major determinant for the success of stem cell therapy-cross-talk between muscle-derived stem cells and host cells. Stem Cell Res Ther. 2011, 2: 31-10.1186/scrt72.
Zarjou A, Kim J, Traylor AM, Sanders PW, Balla J, Agarwal A, Curtis LM: Paracrine effects of mesenchymal stem cells in cisplatin-induced renal injury require heme oxygenase-1. Am J Physiol Renal Physiol. 2011, 300: F254-F262. 10.1152/ajprenal.00594.2010.
Gheisari Y, Ahmadbeigi N, Naderi M, Nassiri SM, Nadri S, Soleimani M: Stem cell-conditioned medium does not protect against kidney failure. Cell Biol Int. 2011, 35: 209-213. 10.1042/CBI20100183.
Lennon DP, Edmison JM, Caplan AI: Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: effects on in vitro and in vivo osteochondrogenesis. J Cell Physiol. 2001, 187: 345-355. 10.1002/jcp.1081.
Hung SC, Pochampally RR, Hsu SC, Sanchez C, Chen SC, Spees J, Prockop DJ: Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS One. 2007, 2: e416-10.1371/journal.pone.0000416.
Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA, Wei L: Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008, 135: 799-808. 10.1016/j.jtcvs.2007.07.071.
Rosova I, Dao M, Capoccia B, Link D, Nolta JA: Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008, 26: 2173-2182. 10.1634/stemcells.2007-1104.
Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS: Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005, 11: 367-368. 10.1038/nm0405-367.
Sorrell JM, Caplan AI: Topical delivery of mesenchymal stem cells and their function in wounds. Stem Cell Res Ther. 2010, 1: 30-10.1186/scrt30.
Acknowledgments
This study was supported by research grants from the National Natural Science Foundation of China (No. 81070569 and No. 81370812), the National Basic Research Program of China 973 Program (No. 2012CB517602 and No. 2012CB517 803), the Research Fund for the Doctoral Program of Ministry of Education of China, the Special Grade of China Postdoctoral Science Foundation (No. 201003463), and the Heilongjiang Postdoctoral Science Research Foundation (No. LBH-Q10028).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
BL designed the research. BL and LX wrote the manuscript and collected and analyzed the data. LX, RC, LP, JM and XC performed research. R-JX contributed to analyzing the data. All authors read and approved the final manuscript.
Electronic supplementary material
13287_2013_389_MOESM1_ESM.jpeg
Additional file 1: is Figure S1 showing light microscopy images of MSCs at 0, 24, 48 and 72 hours of culture with fetal bovine serum (FBS)-free DMEM, examined by MTT and trypan blue staining. (A to D) Light microscopy of MSCs at 0, 24, 48 and 72 hours of culture with FBS-free DMEM. Graph showing MTT cell viability assay (E) and trypan blue staining (F) cultured with FBS-free DMEM at different time points, #P < 0.01 versus the control medium (DMEM not cultured with MSCs). (JPEG 3 MB)
13287_2013_389_MOESM2_ESM.jpeg
Additional file 2: is Figure S2 showing images from animals for histological and immunofluorescent assessments on days 3, 5 and 7 after sham surgery. There were no significant differences between different time points for histological evaluation (a), peritubular capillary loss (b), KI67+ cells (c), CD68+ macrophages (d) and apoptotic cells (e). (JPEG 5 MB)
13287_2013_389_MOESM3_ESM.jpeg
Additional file 3: is Figure S3 showing that no effective results were observed after administration of 500 μl CM to mice. There were no significant differences between the CM and vehicle groups when examined for histological alterations (a), capillary density (b), proliferation of parenchymal cells (c), macrophage infiltration (d) and TUNEL apoptotic cells (e). (JPEG 4 MB)
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
**ng, L., Cui, R., Peng, L. et al. Mesenchymal stem cells, not conditioned medium, contribute to kidney repair after ischemia-reperfusion injury. Stem Cell Res Ther 5, 101 (2014). https://doi.org/10.1186/scrt489
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/scrt489