Abstract
Background
Postinfarction cardiac remodeling presents a compensatory mechanism aimed at mitigating congestive heart failure. It is distinguished by progressive dilatation and hypertrophy of the ventricular chambers, fibrotic alterations, and prolonged apoptosis of cardiomyocytes. The primary objective of this study was to assess the effects of icariin on myocardial fibrosis and ventricular remodeling in rats subjected to myocardial infarction (MI).
Methods
Male Sprague‒Dawley (SD) rats were subjected to randomization and subsequently divided into distinct groups: the control group, the sham group (undergoing sham operation), the MI group (experiencing ligation of the left anterior descending artery), and the icariin group. Within the icariin group, rats were further categorized into three different dose groups based on the administered icariin dosage: the MI30 group (30 mg/kg/day), the MI60 group (60 mg/kg/day), and the MI120 group (120 mg/kg/day). Cardiac function evaluation was carried out using echocardiography. Histological examinations, including hematoxylin and eosin (HE) staining, Masson staining, and immunohistochemistry studies, were conducted 90 days after the occurrence of MI. Additionally, Western blotting was employed to assess TGF‐β1, p-Smad2, and p-Smad3 levels.
Results
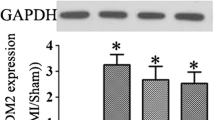
The administration of icariin revealed a noteworthy enhancement in cardiac function among rats afflicted with left anterior descending coronary artery (LAD) ligation. In comparison to the icariin groups, the MI group exhibited reduced EF and FS, along with elevated LVEDD and LVESD. Furthermore, the cardiac fibrosis levels in the MI group rats exhibited a considerable increase compared to those in the icariin group. Notably, the levels of Collagen I, Collagen III, MMP2, and MMP9 were significantly higher in the MI group than in the icariin group, with evident distinctions. Moreover, the expression levels of TGF-β, IL-13, p-Smad2, and p-Smad3 were notably upregulated in the MI group compared to the icariin group.
Conclusions
In an experimental rat model of MI, the administration of icariin resulted in the amelioration of both cardiac function and remodeling processes, operating through the intricate TGF-β1/Smad signaling pathway.
Similar content being viewed by others
Introduction
As the standard of living steadily improves, the aging population is on the rise, and myocardial infarction (MI) stands as the foremost contributor to both morbidity and mortality within the realm of cardiovascular diseases worldwide [1]. While modern reperfusion therapy serves as the most efficacious approach for diminishing infarct size and ameliorating clinical outcomes after MI, heart failure remains the primary cause of death subsequent to MI. The process of heart remodeling following MI is acknowledged as the initial phase leading toward heart failure [2, 3]. Consequently, the reversal of ventricular remodeling emerges as a highly desirable prospect for the treatment of MI. Certain angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs), when administered as long-term treatment, have demonstrated the capacity to attenuate ventricular remodeling [4,5,6]. However, a significant number of patients encounter intolerance to ACEIs/ARBs due to conditions such as renal dysfunction, hypotension, or hyperkalemia, among others. Hence, there exists considerable interest in identifying novel therapeutic agents capable of enhancing ventricular remodeling, thereby addressing this pressing concern.
Myocardial fibrosis and ventricular remodeling have been identified as crucial pathological factors contributing to unfavorable outcomes following MI [7]. Of notable significance, the TGF‐β1/Smad signaling pathway exhibits a close association with both myocardial fibrosis and ventricular remodeling [8, 27,28,29]. TCM compounds are known to comprise a diverse array of Chinese medicinal ingredients that act through various targets, channels, and pathways to address diseases. Among these, icariin, the primary active ingredient derived from the Chinese herbal medicine Epimedium brevicornu Maxim, has garnered considerable interest [30, 31]. Several species of Epimedium can be used to extract it, including Epimedium sagitta, Epimedium pilose, Epimedium Wushan, and Epimedium korean. Icariin has been shown to protect the myocardium from ischemia and reperfusion in previous studies [32,33,34,35]. Icariin has a therapeutic effect on coronary heart disease, but its mechanism remains largely unclear. According to TCM principles, ventricular dysfunction and heart failure arise from Qi deficiency and blood stasis [36, 37]. In this study, icariin therapy exhibited notable improvements in LVEF and FS while concurrently reducing LVEDD and LVESD compared to the MI group. Moreover, this study sheds light on the hitherto unexplored function and mechanism of icariin in mitigating cardiac fibrosis.
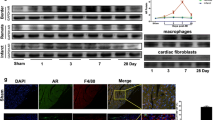
The TGF-β1/Smad signaling pathway assumes a pivotal role in cardiac fibrosis and ventricular remodeling [38]. This study sought to quantify the changes in the expression levels of key molecules within the TGF-β1 signaling pathway. Notably, following MI, there was evident overexpression of TGF-β1 in the myocardium, whereas treatment with icariin resulted in a significant reduction in TGF-β1 in the ischemic myocardium. To validate this hypothesis, Western blotting was performed on M2-type macrophages induced by IL4, revealing that icariin supplementation led to decreased levels of TGF-β1 and Smad2/3. Presently, research concerning the antitumor activity of icariin has emerged as a prominent area of interest. These findings demonstrate that icariin effectively inhibits M2 macrophage polarization, signifying its potential to modulate the tumor microenvironment. Prior studies have already established icariin's ability to inhibit M2 macrophage polarization while promoting M1 macrophage polarization [18, 39]. There is still work to be done to determine whether icariin affects myocardial fibrosis and ventricular remodeling by regulating macrophage polarization.
In conclusion, the present study provides compelling evidence that icariin significantly reduces cardiac fibrosis and ameliorates cardiac function in rats with myocardial infarction. The potential protective effects of icariin are closely associated with modulation of the TGF-β1/Smad signaling pathway. However, it is essential to acknowledge certain limitations within this study. First, the follow-up period for the MI rats was relatively short, thereby precluding the observation of long-term changes in cardiac function. Second, given the intricate molecular mechanisms involved, the precise mechanisms through which icariin confers protective effects on cardiac fibrosis remain incompletely elucidated. Thus, further investigations are warranted to validate and expand upon the present findings.
Limitations of the study
Firstly, the selection of icariin dosage in our study was based on a review of pertinent literature. However, it is noteworthy that there is a dearth of dose–response investigations aimed at determining the most efficacious icariin dosage for achieving optimal outcomes. Additionally, comprehensive long-term safety evaluations for icariin treatment are presently lacking. Secondly, we did not employ TTC staining to validate the myocardial infarction's extent. Lastly, it should be acknowledged that this study was characterized by a limited sample size, an absence of power calculations, and the potential influence of uncontrolled confounding variables.
Conclusion
The findings of the current study suggest that icariin possesses the capacity to enhance cardiac function and mitigate ventricular remodeling in rats following MI. These results underscore icariin's potential as a viable therapeutic target for addressing myocardial injury and ventricular remodeling.
Availability of data and materials
All data and materials utilized in this study are accessible upon reasonable request from the corresponding author.
Abbreviations
- MI:
-
Myocardial infarction
- LAD:
-
Left anterior descending coronary artery
- LVEDD:
-
Left ventricular end-diastolic diameter
- LVESD:
-
Left ventricular end-systolic diameter
- FS:
-
Left ventricular shortening fraction
- EF:
-
Left ventricular ejection fraction
- ACEI:
-
Angiotensin-converting enzyme inhibitors
- ARB:
-
Angiotensin receptor blockers
- RAAS:
-
Renin–angiotensin–aldosterone system
- MMPs:
-
Matrix metalloproteinases
- TCM:
-
Traditional Chinese medicine
- ELISA:
-
Enzyme-linked immunosorbent assay
- SD:
-
Sprague–Dawley
- H&E:
-
Hematoxylin and eosin
- DMSO:
-
Dimethyl sulfoxide;
- ANOVA:
-
One-way analysis of variance
- TGF-β:
-
Transforming growth factor beta
- ECM:
-
Matrix metalloproteinases
- p‐Smad2:
-
Phosphorylated Smad2
- p‐Smad2:
-
Phosphorylated Smad3
- LV:
-
Left ventricular
References
Levitan EB, Muntner P, Chen L, Deng L, Kilgore ML, Becker D, et al. Burden of coronary heart disease rehospitalizations following acute myocardial infarction in older adults. Cardiovasc Drugs Ther. 2016;30(3):323–31.
Asada K, Saito Y, Sato T, Matsumoto T, Yamashita D, Suzuki S, et al. Prognostic value of natriuretic peptide levels and in-hospital heart failure events in patients with acute myocardial infarction. Circ J. 2023;87(5):640–7.
Lin MS, Wang PC, Lin MH, Kuo TY, Lin YS, Chen TH, et al. Acute heart failure with mildly reduced ejection fraction and myocardial infarction: a multi-institutional cohort study. BMC Cardiovasc Disord. 2023;23(1):272.
Park DY, An S, Attanasio S, Jolly N, Malhotra S, Doukky R, et al. Network meta-analysis comparing angiotensin receptor-neprilysin inhibitors, angiotensin receptor blockers, and angiotensin-converting enzyme inhibitors in heart failure with reduced ejection fraction. Am J Cardiol. 2023;187:84–92.
Maizels L, Wasserstrum Y, Fishman B, Segev A, Ben-Nun D, Younis A, et al. Characterization of heart failure patients with reverse left ventricular remodeling postangiotensin receptor blockers/neprilysin inhibitors therapy. ESC Heart Fail. 2022;9(3):1682–8.
Houchen E, Loefroth E, Schlienger R, Proudfoot C, Corda S, Saha S, et al. Hospitalization rates in patients with heart failure and reduced ejection fraction initiating sacubitril/valsartan or angiotensin-converting enzyme inhibitors/angiotensin receptor blockers: a retrospective cohort study. Cardiol Ther. 2022;11(1):113–27.
Bacmeister L, Schwarzl M, Warnke S, Stoffers B, Blankenberg S, Westermann D, et al. Inflammation and fibrosis in murine models of heart failure. Basic Res Cardiol. 2019;114(3):19.
Pisklova M, Osmak G, Favorova O. Regulation of smad signaling pathway by mirnas associated with myocardial fibrosis: In silico analysis of target gene networks. Biochemistry. 2022;87(8):832–8.
Lin P, Tong X, Xue F, Qianru C, **nyu T, Zhe L, et al. Polystyrene nanoplastics exacerbate lipopolysaccharide-induced myocardial fibrosis and autophagy in mice via ros/tgf-beta1/smad. Toxicology. 2022;480: 153338.
Sinpitaksakul SN, Pimkhaokham A, Sanchavanakit N, Pavasant P. Tgf-beta1 induced mmp-9 expression in hnscc cell lines via smad/mlck pathway. Biochem Biophys Res Commun. 2008;371(4):713–8.
Hall MC, Young DA, Waters JG, Rowan AD, Chantry A, Edwards DR, et al. The comparative role of activator protein 1 and smad factors in the regulation of timp-1 and mmp-1 gene expression by transforming growth factor-beta 1. J Biol Chem. 2003;278(12):10304–13.
Derynck R, Zhang YE. Smad-dependent and smad-independent pathways in tgf-beta family signaling. Nature. 2003;425(6958):577–84.
Ikeuchi M, Tsutsui H, Shiomi T, Matsusaka H, Matsushima S, Wen J, et al. Inhibition of tgf-beta signaling exacerbates early cardiac dysfunction but prevents late remodeling after infarction. Cardiovasc Res. 2004;64(3):526–35.
Yeh TC, Ho ST, Hsu CH, Wang JO, Kao S, Su YC, et al. Preoperative use and discontinuation of traditional chinese herbal medicine and dietary supplements in taiwan: a cross-sectional questionnaire survey. Healthcare. 2023;11(11):1605.
Luo H, Zan F, Cui J. Effect of microneedle roller on promoting transdermal absorption of crossbow-medicine liquid via transdermal administration of traditional chinese medicine and the safety of crossbow-medicine needle therapy: an experimental study. J Ethnopharmacol. 2023;317:16751.
Fang J, Zhang Y. Icariin, an anti-atherosclerotic drug from chinese medicinal herb horny goat weed. Front Pharmacol. 2017;8:734.
Wu B, Feng JY, Yu LM, Wang YC, Chen YQ, Wei Y, et al. Icariin protects cardiomyocytes against ischemia/reperfusion injury by attenuating sirtuin 1-dependent mitochondrial oxidative damage. Br J Pharmacol. 2018;175(21):4137–53.
Zheng X, Li D, Li J, Wang B, Zhang L, Yuan X, et al. Optimization of the process for purifying icariin from herba epimedii by macroporous resin and the regulatory role of icariin in the tumor immune microenvironment. Biomed Pharmacother. 2019;118: 109275.
Chen HA, Chen CM, Guan SS, Chiang CK, Wu CT, Liu SH. The antifibrotic and anti-inflammatory effects of icariin on the kidney in a unilateral ureteral obstruction mouse model. Phytomedicine. 2019;59: 152917.
Hao H, Zhang Q, Zhu H, Wen Y, Qiu D, **ong J, et al. Icaritin promotes tumor t-cell infiltration and induces antitumor immunity in mice. Eur J Immunol. 2019;49(12):2235–44.
Wang J, Meng Y, Zhang C, Lu Y, Hu C, Xu K. Delays in first medical contact to primary interventional therapy and left ventricular remodeling in st-segment elevation myocardial infarction. Ir J Med Sci. 2023. https://doi.org/10.1007/s11845-023-03283-z.
Fragasso G. Severe left ventricular dysfunction after acute myocardial infarction: a call for development of adequately targeted treatments. Am J Cardiol. 2023;200:213–4.
El Amrawy AM, Zaghloul SAE, El Sharkawy EM, Sobhy MA. Prognostic value of right ventricular diastolic dysfunction in patients with inferior st-elevated myocardial infarction. Egypt Heart J. 2023;75(1):31.
Frampton J, Ortengren AR, Zeitler EP. Arrhythmias after acute myocardial infarction. Yale J Biol Med. 2023;96(1):83–94.
Demidova MM, Rylance R, Koul S, Dworeck C, James S, Aasa M, et al. Prognostic value of early sustained ventricular arrhythmias in st-segment elevation myocardial infarction treated by primary percutaneous coronary intervention: a substudy of validate-swedeheart trial. Heart Rhythm O2. 2023;4(3):200–6.
Wang L, Du A, Lu Y, Zhao Y, Qiu M, Su Z, et al. Peptidase inhibitor 16 attenuates left ventricular injury and remodeling after myocardial infarction by inhibiting the hdac1-wnt3a-beta-catenin signaling axis. J Am Heart Assoc. 2023;12(10): e028866.
Cheang I, Liao S, Zhu Q, Ni G, Wei C, Jia Z, et al. Integrating evidence of the traditional chinese medicine collateral disease theory in prevention and treatment of cardiovascular continuum. Front Pharmacol. 2022;13: 867521.
Hao P, Jiang F, Cheng J, Ma L, Zhang Y, Zhao Y. Traditional chinese medicine for cardiovascular disease: Evidence and potential mechanisms. J Am Coll Cardiol. 2017;69(24):2952–66.
Gao Z, Li S, Shang Q, Jiao Y, Zhou X, Fu C, et al. Complex networks approach for analyzing the correlation of traditional chinese medicine syndrome evolvement and cardiovascular events in patients with stable coronary heart disease. Evid Based Complement Alternat Med. 2015;2015: 824850.
Yuan JY, Tong ZY, Dong YC, Zhao JY, Shang Y. Research progress on icariin, a traditional chinese medicine extract, in the treatment of asthma. Allergol Immunopathol. 2022;50(1):9–16.
Chai Y, Ji S, Zhang G, Wu Y, Yin X, Liang D, et al. Determination of icariin in chinese traditional medicine by capillary zone electrophoresis. Biomed Chromatogr. 1999;13(5):373–5.
Ke Z, Liu J, Xu P, Gao A, Wang L, Ji L. The cardioprotective effect of icariin on ischemia-reperfusion injury in isolated rat heart: potential involvement of the pi3k-akt signaling pathway. Cardiovasc Ther. 2015;33(3):134–40.
Meng X, Pei H, Lan C. Icariin exerts protective effect against myocardial ischemia/reperfusion injury in rats. Cell Biochem Biophys. 2015;73(1):229–35.
Zhai M, He L, Ju X, Shao L, Li G, Zhang Y, et al. Icariin acts as a potential agent for preventing cardiac ischemia/reperfusion injury. Cell Biochem Biophys. 2015;72(2):589–97.
Zhang F, Hu Y, Xu X, Zhai X, Wang G, Ning S, et al. Icariin protects against intestinal ischemia-reperfusion injury. J Surg Res. 2015;194(1):127–38.
Sun S, Wei Y, Zeng X, Yuan Y, Wang N, An C, et al. Circulating cd14(+)hla-dr(−/low) myeloid-derived suppressor cells as potential biomarkers for the identification of psoriasis tcm blood-heat syndrome and blood-stasis syndrome. Evid Based Complement Alternat Med. 2020;2020:4582459.
**ng M, Luo Y, Kuai L, Ru Y, Ding X, Yan X, et al. DNA methylation expression profile of blood heat syndrome and blood stasis syndrome in tcm psoriasis. Evid Based Complement Alternat Med. 2022;2022:9343285.
Hojo Y, Saito T, Kondo H. Role of apoptosis in left ventricular remodeling after acute myocardial infarction. J Cardiol. 2012;60(2):91–2.
Song L, Chen X, Mi L, Liu C, Zhu S, Yang T, et al. Icariin-induced inhibition of sirt6/nf-kappab triggers redox mediated apoptosis and enhances antitumor immunity in triple-negative breast cancer. Cancer Sci. 2020;111(11):4242–56.
Funding
This research was supported by the Science Research Foundation of Yunnan Provincial Department of Education Project (NO. 2022J0017), Medical Reserve Talent Training Program of Yunnan Provincial Health Commission of China (NO. H-2017019), and Yunnan Provincial Program for the Cultivation of High-level Innovative Health Talent (NO. YNWR-MY-2020-024).
Author information
Authors and Affiliations
Contributions
The study was conceptualized by Ji Jia, **ng-an Zhao, Si-ming Tao, and Jun-wen Wang. The experimentation was conducted by Ji Jia, **ng-an Zhao, Si-ming Tao, Rong-liang Zhang, and **-tao Li. Ji Jia, **ng-an Zhao, and Si-ming Tao made significant contributions to the data analysis and manuscript preparation. Ji Jia and Si-ming Tao performed the data analyses and authored the manuscript. Hua-lei Dai, **n-** Zhang, Ming-hua Han, Bei Yang, and Yu Li provided constructive discussions and assistance during the analysis process.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The ethics committee of the Animal Research Committee of the Yunnan University College of Medicine reviewed and approved the study (approval number YNU20220333).
Consent for publication
This manuscript is approved by all authors for publication.
Informed consent
Not applicable.
Competing interests
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jia, J., Zhao, Xa., Tao, Sm. et al. Icariin improves cardiac function and remodeling via the TGF-β1/Smad signaling pathway in rats following myocardial infarction. Eur J Med Res 28, 607 (2023). https://doi.org/10.1186/s40001-023-01588-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40001-023-01588-4