Abstract
Objective
This study aimed to develop and apply a nomogram with good accuracy to predict the risk of CRAB infections in neuro-critically ill patients. In addition, the difficulties and expectations of application such a tool in clinical practice was investigated.
Methods
A mixed methods sequential explanatory study design was utilized. We first conducted a retrospective study to identify the risk factors for the development of CRAB infections in neuro-critically ill patients; and further develop and validate a nomogram predictive model. Then, based on the developed predictive tool, medical staff in the neuro-ICU were received an in-depth interview to investigate their opinions and barriers in using the prediction tool during clinical practice. The model development and validation is carried out by R. The transcripts of the interviews were analyzed by Maxqda.
Results
In our cohort, the occurrence of CRAB infections was 8.63% (47/544). Multivariate regression analysis showed that the length of neuro-ICU stay, male, diabetes, low red blood cell (RBC) count, high levels of procalcitonin (PCT), and number of antibiotics ≥ 2 were independent risk factors for CRAB infections in neuro-ICU patients. Our nomogram model demonstrated a good calibration and discrimination in both training and validation sets, with AUC values of 0.816 and 0.875. Additionally, the model demonstrated good clinical utility. The significant barriers identified in the interview include “skepticism about the accuracy of the model”, “delay in early prediction by the indicator of length of neuro-ICU stay”, and “lack of a proper protocol for clinical application”.
Conclusions
We established and validated a nomogram incorporating six easily accessed indicators during clinical practice (the length of neuro-ICU stay, male, diabetes, RBC, PCT level, and the number of antibiotics used) to predict the risk of CRAB infections in neuro-ICU patients. Medical staff are generally interested in using the tool to predict the risk of CRAB, however delivering clinical prediction tools in routine clinical practice remains challenging.
Similar content being viewed by others
Introduction
Acinetobacter baumannii (A. baumannii) is a significant pathogen responsible for nosocomial infections in healthcare settings [1]. The prevalence of carbapenem-resistant A. baumannii (CRAB) is on the rise globally due to A. baumannii's complex drug resistance and range of virulence factors, as well as the improper use of antibiotics [2, 3]. For example, the resistance rate of A.baumannii to carbapenems is as high as 88% in Europe and 85% in Latin America [4, 5]. A recent study reported resistance rates of A. baumannii to imipenem and meropenem in the intensive care unit (ICU) of a general hospital in Greece, reaching as high as 100% and 98.91%, respectively [6]. As bacterial resistance mechanisms are evolving and limiting available therapeutic options [7], this has resulted in CRAB infections are linked with higher mortality rates, longer stays in the ICU, and increased patient costs [8,9,10]. For example, among patients infected with CRAB who did not receive appropriate empiric antimicrobial therapy, the overall mortality rate was 86.1% [11].
Critically ill patients are susceptible to infection due to delayed immune responses and the use of invasive devices [12]. For instance, a recent study found that CRAB strains were prevalent in 71.4% of critically ill patients admitted to ICU [13]. Identification of risk factors for CRAB infections is crucial for the early implementation of an appropriate therapy and for improving clinical outcomes. Reported risk factors associated with CRAB infections include age, sex, malignancy, mechanical ventilation, hemodialysis, length of ICU stay, transfusion, and previous use of carbapenems and aminoglycosides [20]. Therefore, it is crucial to accurately predict the occurrence of CRAB infections early in patients in the neuro-ICU. A risk prediction model for CRAB infections for patients in the neuro-ICU is unavailable. Only a few studies have investigated the difficulties of medical staff in applying such prediction tools in clinical practice.
Therefore, we aimed to develop and validate a nomogram to predict the risk of CRAB infections in neuro-critically ill patients. Moreover, we aimed to provide information about the barriers to the use of such risk prediction tools in the clinical setting from the clinical staff side.
Materials and methods
Quantitative study
Study design
A retrospective cohort study was conducted on neuro-ICU at a hospital in Yangzhou city, Jiangsu Province, China between January to December 2019. Data from neuro-ICU patients were randomized into training and validation sets at a 7:3 ratio. A nomogram from the training test was created using independent risk factors to predict CRAB infections in the neuro-ICU.
Ethics statement
This study was approved by the Medical Ethics Committee of the Clinical Medical College of Yangzhou University(2023ky129). Informed consent was waived because the study was conducted retrospectively and no interventions were applied. This study was conducted in accordance with the standards set by the Declaration of Helsinki. To protect the privacy of participants, all personal information was de-identified prior to analysis.
Study population and definitions
Patients qualified for enrollment if they were aged ≥ 18 years. The following exclusion criteria were used: (1) the length of stay in the neuro-ICU was < 24 h; (2) CRAB was detected before the patient entered the neuro-ICU or within the first 48 h of admission in the neuro-ICU or 72 h after leaving the neuro-ICU. CRAB strains were defined as resistant if they showed resistance to at least one carbapenem antibiotic [21]. The diagnosis of nosocomial infection refers to the “Diagnostic Criteria for Hospital Infection (Trial)” issued by the Chinese Ministry of Health [22]. Patients with CRAB infections constituted the CRAB infections group, and those without CRAB infections constituted the non-CRAB infections group.
Strain collection, strain identification and susceptibility testing
Eligible samples of sputum, urine, cerebrospinal fluid, blood, and catheters were collected and sent to the microbiology laboratory after the clinical nurse ensured that the samples were adequate and free of contamination. The specimens were then inoculated on culture plates and incubated at 37 °C for 48 h. The strains were identified using a French Mérieux VITEK 2 Compact fully automated microbiology analyzer. Drug sensitivity testing was performed by paper diffusion method and the isolates were characterized as sensitive, intermediary or resistant according to Clinical and Laboratory Standards Institute guidelines. All bacterial isolation, culture and identification were done in the microbiology department of our hospital.
Selection of predictive variables
To include all potential predictive variables, we first searched extensively on published studies on risk factors for CRAB infections in patients admitted to the ICU, particularly through systematic review and meta-analysis. Based on the search, we assessed the available variables in electronic medical records in the study hospital. Subsequently, we organized a panel discussion with doctors from both ICUs and neuro-ICU experts to finally identify variables included in our analysis (Table S1).
Data collection
Demographic and clinical data were collected from the included patients by researcher using hospital health information system. The following data, which may be related to CRAB infections based on the literature review and clinical experience, were extracted: patients’ general characteristics, including age, gender, comorbidities, and the length of stay in neuro-ICU; laboratory examination, including the levels of albumin, red blood cell (RBC) count, platelets, leucocyte count, procalcitionin (PCT) within the first 48 h in the neuro-ICU; treatment, including surgery, and number of antibiotics. The detailed definition of candidate variables are described in Supplement.
Handling of missing data
Our approach was tailored according to the proportion of missing data to address missing values of variables. For instance, where the proportion remained below 5%, we adopted the mean filling method as a suitable solution. Conversely, when the proportion exceeded this threshold, we employed the multiple interpolation method to handle the missing data. In cases where the number of missing variable items surpassed 10% of the total variable items, we adopted the case deletion method [23].
Statistical analysis
Categorical variables were presented as counts and percentages (%), while continuous variables were presented as either mean ± standard deviation or median and interquartile range. The independent samples t-test was used to compare two groups of parametric values, and the Mann–Whitney U test was used to compare two groups of nonparametric values. Categorical variables were compared using the chi-square test. Following the univariate analysis, we conducted multivariate logistic regression analysis to determine the odds ratio (OR) and 95% confidence interval (CI) of independent variables. The variables selected for the multivariate model were chosen based on their physiological relevance and statistical significance in the univariate analysis. A threshold P value of 0.25 was used in the selection process. All statistical tests were two-tailed and a significance level of 0.05 was used for the multivariate analysis. The above statistical tests were performed using Stata15 software.
A predictive nomogram model was formulated based on the results of the multivariate analysis of the training test, which allowed us to better predict CRAB infections. We evaluated the accuracy of the nomogram using an internal validation cohort. The predictive capacity of the nomogram was quantified by calculating the area under the receiver operating characteristic (ROC) curve (AUC). Accuracy, sensitivity, specificity, detection rate, positive predictive value (PPV), and negative predictive value (NPV) were used to evaluate the performance of the nomogram model. Evaluation of calibration accuracy through calibration plot. In the calibration plot, the Brier score provided the difference between the probabilistic predictions and the true results. It ranges from 0 to 1, with scores closer to 0 indicating better predictions. Calibration slope is preferred to assess calibration and is used to evaluate the agreement between observed and predicted values, with values closer to 1 indicating better performance [24]. The calibration-in-the-large was calculated as the intercept coefficient of the logistic regression with the linear predictor as an offset; zero was the ideal value [25]. Furthermore, we used decision curve analysis (DCA) to assess the effectiveness in a clinical environment. During the development and validation phase, all analyses were performed using R software (version 4.2.0, https://www.R-project.org).
Qualitative study
We conducted a in-depth interview to explore the attitudes and barriers of clinical medical staff in using such prediction tools in neuro-ICU context. Participants were selected through convenience and purposive sampling. Data collection and analysis were carried out simultaneously until data saturation was reached. The interviews were transcribed verbatim within 24 h of the interview and reviewed for accuracy by the interviewer. All materials, including interviews, original transcripts, and data analysis, were written in Chinese.
The study utilized a semi-structured format with open-ended questions, which was developed by the research team based on relevant literature and an intensive group involving clinical experts and epidemiologistis. Illustrative questions were: “what is your overall opinion regarding clinical risk prediction model?”; “what are the difficulties you may encounter in using this model in your clinical work?” Probing questions, such as “please elaborate on that,” were used to elicit more information. The transcripts of the interviews were analyzed by Maxqda.
Results
Quantitative results
Basic characteristics
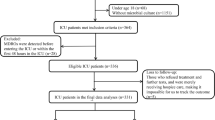
Among the 632 patients admitted to the neuro-ICU from January to December 2019, 544 patients were eventually eligible and included in the statistical analysis (Fig. 1). Table 1 provides a summary of the main demographic and clinical characteristics of the participants.
Incidence of CRAB infections
The total incidence of CRAB infections was 8.63% (47/544), with an incidence of 9.29% (37/398) in the training set and 6.84% (10/146) in the validation set. The CRAB strains were first detected from sputum in 41 of the infected patients, in urine culture in five patients, and from other sources in one patient.
Results of univariate and multivariable analyses
Univariate analysis indicated that the male, low GCS score, long length of stay in neuro-ICU, diabetes, low ALB level, low RBC count, high PCT level, high urea value, high PH value, low blood glucose value, and the number of types of antibiotics ≥ 2 were significantly associated with CRAB infections (Table 2). Results of multivariate analysis demonstrated that the following six variables were independent predictors for CRAB infections, including the male sex (OR:3.11; 95% CI: 1.13–8.54, P = 0.027), long stay in neuro-ICU (OR:1.07; 95% CI:1.02–1.11, P = 0.001), diabetes (OR:3.05, 95% CI:1.19–7.84, P = 0.020), low RBC (OR:0.54; 95% CI: 0.30–0.98, P = 0.043), high PCT level (OR:1.07; 95% CI:1.00–1.15, P = 0.049), and the number of antibiotics ≥ 2 (OR: 3.92; 95%CI:1.65–9.30, P = 0.002).
Development of the nomogram
We developed a nomogram, as shown in Fig. 2A using independent risk factors to predict the risk of CRAB infections in neuro-ICU patients. To demonstrate the use of the nomogram, consider a male patient who was admitted for 10 days, was on two or more antibiotics, had diabetes, and his PCT measurement was 2.5 ng/ml with an RBC count of 5 × 1012/L. Using the legend, the total score for this patient was 155.5, which corresponded to a CRAB infection risk of approximately 31%.
Validation of the nomogram
In the training cohort, the AUC achieved using the nomogram model was 0.816 (95% CI: 0.734–0.896). In the validation cohort, the predictive power of the nomogram was confirmed internally. The AUC of the nomogram was 0.875 (95% CI 0.747–0.944), indicating a good discrimination. In addition, Fig. 3 shows that the AUCs of both groups indicate that the nomogram model outperforms individual risk factors in terms of AUC. Table 3 shows that the accuracy, sensitivity, detection rate, and PPV of the nomogram model for predicting CRAB infections are better, with an accuracy of 0.899, a sensitivity of 0.983, and a PPV of 0.913. These values indicate that the model can well recognize neurocritical patients who develop CRAB infections. The calibration plot showed that apparent and bias-corrected lines deviated slightly from the ideal line, indicating that the predictions were in line with the actual results. We evaluated the nomogram model. Its Brier score was 0.047; calibration slope was 1, and calibration-in-the-large was 0, indicating that the model had good prediction accuracy (Fig. 4).
Evaluation of the clinical applicability of the nomogram
The DCA of the validation set revealed that using this predictive nomogram was more favorable for patients when the threshold probability was set between 30 and 82%. This is in contrast to the extreme approaches of detecting CRAB infections in all patients or no detection. These findings indicate that the present model offers a greater net benefit for predicting CRAB infections development across a relatively wide Fig. 5.
Qualitative results
A total of 10 medical staff, comprising 3 nurses and 7 physicians (5 females and 5 males), aged between 26 and 60 years, participated in the qualitative interviews. Their professional tenures in neuro-ICU ranged from 2 and 19 years. Three major barriers involving in the use of nomogram models identified here include “skepticism about the accuracy of the model”, “delay in early prediction by the indicator of length of neuro-ICU stay”, and “lack of a proper protocol for clinical application”.
The medical staff mainly concerned about the accuracy of the model when considering the use of the model into clinical practice. They expected the prediction models could be fully validated before its use. Importantly, over half of the participants mentioned that “the length of stay in the neuro-ICU” although is an important predictor, it is often recorded in a rather late stage. Therefore, it may not contribute to an early prediction. Finally, the medical staff stated that currently there is no acknowledged protocol in using the prediction models in the clinical practice. For example, considering when and how to use and interpret the model, a proper guidance may needed.
Discussion
In this study, we developed a nomogram to predict the risk of CRAB infections in neuro-critically ill patients. The model is based on easily obtainable indicators such as the length of neuro-ICU stay, male, diabetes, RBC, PCT level, and the number of antibiotics used. We validated the prediction model and found it to have good overall predictive value. It may serve as a useful tool for medical staff to predict the risk of CRAB infections among neuro-ICU patients.
Individualized predictive models can help clinical staff in the neuro-ICU to make early predictions regarding the onset of CRAB infection and implement targeted interventions. The model established had high sensitivity and low specificity, reflecting that the sensitivity and specificity were inversely proportional. The higher the sensitivity, the lower the specificity, and vice versa [26]. A higher sensitivity of the predictive model is crucial to predict CRAB infections in the neuro-ICU. Lower specificity of the predictive model may lead to some false-positive results [27], However, it can benefit critically ill patients as the screening and treatment measures usually follow in critical care contexts.
The study found that patients admitted to neuro-ICU with diabetes have an increased risk of CRAB infections. This may be due to the negative impact of diabetes on the immune system, which hinders humoral and cell-mediated immune responses, thus making it more difficult to eradicate pathogens [28]. A meta-analysis also suggested that individuals with type 2 diabetes are more likely to develop infections caused by resistant bacteria compared with those without diabetes [29]. Glucose control is a hot topic in the field of neurological care, given that neuro-critically ill patients are very sensitive to hyperglycemia [30]. Strict glycemic control in the past was considered a standard therapeutic intervention, as a large clinical trial by van den Berghe et al. in 2001 suggested that strict glucose control (< 110 mg/dL) reduces mortality in critically ill patients [31]. However, the NICE-SUGAR study published in 2009 showed that strict glucose control led to increased mortality secondary to hypoglycemia [32]. Later, a systematic review and meta-analysis of 16 clinical trials on optimal glycemic control in neurocritical care patients, revealed that strict glycemic control (70–140 mg/dL) had no effect on patient mortality but it increased the incidence of hypoglycemia [33]. Until recently, the ADA stated that a blood glucose level of 110–140 mg/dL may be appropriate if significant hypoglycemia can be avoided [34].
Our study has further highlighted that using more than two antibiotics is associated with the risk of CRAB infections. A previous study [35] suggested that three or more antibiotic classes were a risk factor for multidrug-resistant A. baumannii infections. This might be attributed to the fact that combination antibiotic treatment causes dysbiosis in the microbiota, whereby the majority of susceptible bacteria are eradicated, while the antibiotic-resistant strains survive [36]. Additionally, inappropriate combination antibiotic treatment can create selective pressure, thus increasing the likelihood of antibiotic-resistant infection and promoting the selection of drug-resistant bacteria [37]. Substantial debate about the differences in resistance when patients with infections are treated with combination antibiotics versus monotherapy exists. In a large retrospective study, Kosmidis et al. reported that compared to patients with nosocomial pneumonia and patients in the ICU who received combination antibiotic therapy and those treated with monotherapy were more likely to develop drug resistance [38]. However, not all studies support combination therapy to prevent drug resistance. In a prospective randomized trial of 280 adults with severe bacterial infections, Cometta et al. showed that patients treated with imipenem in combination with netimicin developed resistance more frequently than patients treated with imipenem monotherapy [39]. A subsequent meta-analysis of eight clinical trials showed that aminoglycoside/beta-lactam combination therapy had no beneficial effect on the development of antimicrobial resistance in patients with severe infections compared to beta-lactam monotherapy [40]. In general, the use of combination therapy or monotherapy should be determined based on the case, the severity of the disease, the likely pathogenic microorganisms, and local microbiome and resistance patterns.
Higher PCT levels are significant indicators of CRAB infections. PCT is a well-known marker of inflammation that rises rapidly in response to pro-inflammatory stimuli caused by bacteria, and its upregulation suggests the presence of a systemic bacterial infection [41]. Most studies affirm the value of PCT as an effective marker for early diagnosis of infection in critically ill patients [42, 43]. The results of the study further suggested that health professionals should be considered as a sign of increased susceptibility to CRAB infections when patients are in a state of a higher PCT level at the time of admission to the neuro-ICU. However, significant differences in the results of previous studies evaluating PCT for the prediction of bacterial infections exist. One study showed that significantly elevated PCT levels (1.2 ~ 3.6 ng/mL) may be a biomarker for the diagnosis of Wegener’s granulomatous bacterial infections [44], and another study showed that PCT levels > 0.22 ng/mL hint at bacterial infection in patients with vasculitis [45]. This may be related to specific patient groups and different etiologies of infection. Clinicians can perform PCT testing regularly to reduce the risk of CRAB infections.
The clinical use of the predictive model established in this study lacked external validation despite having a good performance and being well-received by medical personnel. External validation should be planned early, as it probably requires data from collaborators and datasets from other centers in different settings. For example, the prevalence of CRAB strains may vary in patients admitted to the ICU across different provinces in China, ranging from 10% in Jiangsu province to 70% in Hunan province [46]. Therefore, the model’s predictive accuracy established could be considered for further testing in these settings. Data from some large, openly, accessible databases, such as the MIMIC-IV database, could also be considered a source for external validation [47, 48].
The medical staff is mainly concerned about the application of the predictive model in their clinical practice. In the Shunde Hospital in Guangdong province, the researchers and clinical staff used an individualized artificial intelligence survival predictive system to generate individual survival curves for patients with lung adenocarcinoma [49]. This artificial intelligence predictive system could directly convey treatment benefits by comparing individual mortality risk curves under different treatment conditions. To promote the easy use of the risk predictive models, freely available, high-quality mortality risk prediction smartphone applications have been used by healthcare professionals to make evidence-based decisions in critical care environment [50]. Integrating well-performed predictive models into the system may promote the use of predictive models in primary care practice. For example, a study in New Zealand integrated a web-based decision support system with primary care electronic medical record software and found that these computerized risk prediction tools increased user visits [51].
Strengths and limitations
Although the variables used in the constructed model are easy to measure and showed good discrimination and calibration in both the training and validation sets, some limitations should be admitted. Firstly, this is a retrospective study that may affect the stability of the CRAB infections prediction model compared to the existing predictions. Second, although the data was from one large tertiary hospital in central China, their ability to represent the general population was limited, especially considering geographical variations in CRAB strains and antibiotic resistance patterns. Therefore, future studies with multiple centers and larger populations from different regions should be conducted to improve the clinical generalizability of the model. Lastly, we only qualitatively explored the opinions and barriers to using the CRAB predictive models established in this study, and we did not conduct surveys to quantify skepticism, perceived utility, and other barriers among a broader range of medical staff, which could complement the qualitative findings and inform more targeted interventions.
Conclusion
The study established a nomogram to predict CRAB infections in neuro-ICU patients with good accuracy. The indicators of the nomogram included the length of neuro-ICU stay, male, diabetes, RBC count, PCT level, and number of antibiotics ≥ 2. The model established here may work as an important tool for early detection and prevention of CRAB infections in neuro-critically ill patients. Medical staff are generally interested in using the tool to predict the risk of CRAB, however, delivering clinical prediction tools in routine clinical practice remains challenging.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- CRAB:
-
Carbapenem-resistant Acinetobacter baumannii
- A.baumannii:
-
Acinetobacter baumannii
- ICU:
-
Intensive care unit
- Neuro-ICU:
-
Neurointensive care unit
- HAIs:
-
Healthcare-associated infections
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
- DCA:
-
Decision curve analysis
- SD:
-
Standard deviation
- IQR:
-
Interquartile range
- INR:
-
International Normalized Ratio
- GCS:
-
Glasgow coma score
- BMI:
-
Body mass index
- RBC:
-
Red blood cell
- WBC:
-
White blood cell
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate transaminase
- PCT:
-
Procalcitonin
- PPV:
-
Positive Predictive Value
- NPV:
-
Negative Predictive Value
References
Chen SJ, Chao TF, Chiang MC, et al. Predictors of mortality in surgical patients with Acinetobacter baumannii bacteremia. J Microbiol Immunol Infect. 2011;44(3):209–14. https://doi.org/10.1016/j.jmii.2011.01.017.
Zhu Y, Zhang X, Wang Y, et al. Insight into carbapenem resistance and virulence of Acinetobacter baumannii from a children's medical centre in eastern China. Ann Clin Microbiol Antimicrob. 2022;21(1):47. Published 2022 Nov 5. https://doi.org/10.1186/s12941-022-00536-0.
Lee MH, Chen TL, Lee YT, et al. Dissemination of multidrug-resistant Acinetobacter baumannii carrying BlaOxA-23 from hospitals in central Taiwan. J Microbiol Immunol Infect. 2013;46(6):419–24. https://doi.org/10.1016/j.jmii.2012.08.006.
Nutman A, Glick R, Temkin E, et al. A case-control study to identify predictors of 14-day mortality following carbapenem-resistant Acinetobacter baumannii bacteraemia. Clin Microbiol Infect. 2014;20(12):O1028–34. https://doi.org/10.1111/1469-0691.12716.
Gales AC, Castanheira M, Jones RN, Sader HS. Antimicrobial resistance among Gram-negative bacilli isolated from Latin America: results from SENTRY Antimicrobial Surveillance Program (Latin America, 2008–2010). Diagn Microbiol Infect Dis. 2012;73(4):354–60. https://doi.org/10.1016/j.diagmicrobio.2012.04.007.
Feretzakis G, Loupelis E, Sakagianni A, et al. A 2-Year Single-Centre Audit on Antibiotic Resistance of Pseudomonas aeruginosa, Acinetobacter baumannii and Klebsiella pneumoniae Strains from an Intensive Care Unit and Other Wards in a General Public Hospital in Greece. Antibiotics (Basel). 2019;8(2):62. https://doi.org/10.3390/antibiotics8020062.
Feretzakis G, Sakagianni A, Loupelis E, et al. Using Machine Learning to Predict Antimicrobial Resistance of Acinetobacter Baumannii, Klebsiella Pneumoniae and Pseudomonas Aeruginosa Strains. Stud Health Technol Inform. 2021;281:43–7. https://doi.org/10.3233/SHTI210117.
Zhen X, Stålsby Lundborg C, Sun X, Gu S, Dong H. Clinical and Economic Burden of Carbapenem-Resistant Infection or Colonization Caused by Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii: A Multicenter Study in China. Antibiotics (Basel). 2020;9(8):514. https://doi.org/10.3390/antibiotics9080514.
Pogue JM, Zhou Y, Kanakamedala H, Cai B. Burden of illness in carbapenem-resistant Acinetobacter baumannii infections in US hospitals between 2014 and 2019. BMC Infect Dis. 2022;22(1):36. https://doi.org/10.1186/s12879-021-07024-4.
Zhen X, Chen Y, Hu X, et al. The difference in medical costs between carbapenem-resistant Acinetobacter baumannii and non-resistant groups: a case study from a hospital in Zhejiang province, China. Eur J Clin Microbiol Infect Dis. 2017;36(10):1989–94. https://doi.org/10.1007/s10096-017-3088-3.
Du X, Xu X, Yao J, et al. Predictors of mortality in patients infected with carbapenem-resistant Acinetobacter baumannii: A systematic review and meta-analysis. Am J Infect Control. 2019;47(9):1140–5. https://doi.org/10.1016/j.ajic.2019.03.003.
Jiang Y, Ding Y, Wei Y, Jian C, Liu J, Zeng Z. Carbapenem-resistant Acinetobacter baumannii: A challenge in the intensive care unit. Front Microbiol. 2022;13:1045206. Published 2022 Nov 10. https://doi.org/10.3389/fmicb.2022.1045206.
Liu C, Chen K, Wu Y, et al. Epidemiological and genetic characteristics of clinical carbapenem-resistant Acinetobacter baumannii strains collected countrywide from hospital intensive care units (ICUs) in China. Emerg Microbes Infect. 2022;11(1):1730–41. https://doi.org/10.1080/22221751.2022.2093134.
Niu T, **ao T, Guo L, et al. Retrospective comparative analysis of risk factors and outcomes in patients with carbapenem-resistant Acinetobacter baumannii bloodstream infections: cefoperazone-sulbactam associated with resistance and tigecycline increased the mortality. Infect Drug Resist. 2018;11:2021–30. https://doi.org/10.2147/IDR.S169432.
Liu Y, Wang Q, Zhao C, et al. Prospective multi-center evaluation on risk factors, clinical characteristics and outcomes due to carbapenem resistance in Acinetobacter baumannii complex bacteraemia: experience from the Chinese Antimicrobial Resistance Surveillance of Nosocomial Infections (CARES) Network. J Med Microbiol. 2020;69(7):949–59. https://doi.org/10.1099/jmm.0.001222.
Djordjevic ZM, Folic MM, Folic ND, Gajovic N, Gajovic O, Jankovic SM. Risk factors for hospital infections caused by carbapanem-resistant Acinetobacter baumannii. J Infect Dev Ctries. 2016;10(10):1073–80. https://doi.org/10.3855/jidc.8231.
Baran G, Erbay A, Bodur H, et al. Risk factors for nosocomial imipenem-resistant Acinetobacter baumannii infections. Int J Infect Dis. 2008;12(1):16–21. https://doi.org/10.1016/j.ijid.2007.03.005.
Maslove DM, Lamontagne F, Marshall JC, Heyland DK. A path to precision in the ICU. Crit Care. 2017;21(1):79. https://doi.org/10.1186/s13054-017-1653-x.
China Neurosurgical Critical Care Specialist Council (CNCCSC), Zhao JZ, Zhou DB, et al. The experts consensus for patient management of neurosurgical critical care unit in China (2015). Chin Med J (Engl). 2015;128(8):1252–67. https://doi.org/10.4103/0366-6999.156146.
Abulhasan YB, Abdullah AA, Shetty SA, Ramadan MA, Yousef W, Mokaddas EM. Health Care-Associated Infections in a Neurocritical Care Unit of a Develo** Country. Neurocrit Care. 2020;32(3):836–46. https://doi.org/10.1007/s12028-019-00856-8.
Bassetti M, Peghin M, Pecori D. The management of multidrug-resistant Enterobacteriaceae. Curr Opin Infect Dis. 2016;29(6):583–94. https://doi.org/10.1097/QCO.0000000000000314.
Ministry of Health of the People's Republic of China. Diagnostic criteria for hospital-acquired infections (for trial implementation) . Chinese Medical Journal. 2001;81: 314–320.
Deng JX, Shan L B, He DQ, et al. Processing Method of Missing Data and Its Develo** Tendency. Stat Decis. 2019;35(23):28–34. https://doi.org/10.13546/j.cnki.tjyjc.2019.23.005.
Semenkovich TR, Yan Y, Subramanian M, et al. A Clinical Nomogram for Predicting Node-positive Disease in Esophageal Cancer. Ann Surg. 2021;273(6):e214–21. https://doi.org/10.1097/SLA.0000000000003450.
Serrano AB, Gomez-Rojo M, Ureta E, et al. Preoperative clinical model to predict myocardial injury after non-cardiac surgery: a retrospective analysis from the MANAGE cohort in a Spanish hospital. BMJ Open. 2021;11(8):e045052. https://doi.org/10.1136/bmjopen-2020-045052. Published 2021 Aug 4.
Yue Y, Shen M, Liu X, et al. Whole-genome sequencing-based prediction and analysis of antimicrobial resistance in Yersinia enterocolitica from Ningxia. China Front Microbiol. 2022;13: 936425. https://doi.org/10.3389/fmicb.2022.936425.
Dankers F, Traverso A, Wee L, et al. Prediction Modeling Methodology [M]//Kubben P, Dumontier M, Dekker A. Fundamentals of Clinical Data Science. Cham (CH); Springer Copyright 2019, The Author(s). 2019: 101–20.
Lin CY, Chen YM, Lin MC, et al. Risk factors of multidrug-resistant Acinetobacter baumannii recurrence after successful eradication in ventilated patients. Biomed J. 2016;39(2):130–8. https://doi.org/10.1016/j.bj.2015.07.001.
Carrillo-Larco RM, Anza-Ramírez C, Saal-Zapata G, et al. Type 2 diabetes mellitus and antibiotic-resistant infections: a systematic review and meta-analysis. J Epidemiol Community Health. 2022;76(1):75–84. https://doi.org/10.1136/jech-2020-216029.
Kramer AH, Roberts DJ, Zygun DA. Optimal glycemic control in neurocritical care patients: a systematic review and meta-analysis. Crit Care. 2012;16(5):R203. https://doi.org/10.1186/cc11812.
van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345(19):1359–67. https://doi.org/10.1056/NEJMoa011300.
NICE-SUGAR Study Investigators, Finfer S, Chittock DR, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283–97. https://doi.org/10.1056/NEJMoa0810625.
Kanji S, Jones E, Goddard R, Meggison HE, Neilipovitz D. Efficiency and safety of a standardized protocol for intravenous insulin therapy in ICU patients with neurovascular or head injury. Neurocrit Care. 2010;12(1):43–9. https://doi.org/10.1007/s12028-009-9275-z.
Professional Practice Committee for the Standards of Medical Care in Diabetes-2016. Diabetes Care. 2016;39 Suppl 1:S107-S108. https://doi.org/10.2337/dc16-S018.
Wang P, Song QM. Establishment of a line graph model for predicting the risk of multiple drug resistance of Acinetobacter baumannii in patients with severe pneumonia. China. Antibiotics Miscellaneous. 2022;47:399–04. https://doi.org/10.13461/j.cnki.cja.007207.
Liao Q, Feng Z, Lin H, et al. Carbapenem-resistant gram-negative bacterial infection in intensive care unit patients: Antibiotic resistance analysis and predictive model development. Front Cell Infect Microbiol. 2023;13:1109418. https://doi.org/10.3389/fcimb.2023.1109418.
Huang H, Chen B, Liu G, et al. A multi-center study on the risk factors of infection caused by multi-drug resistant Acinetobacter baumannii. BMC Infect Dis. 2018;18(1):11. https://doi.org/10.1186/s12879-017-2932-5.
Kosmidis J, Koratzanis G. Emergence of resistant bacterial strains during treatment of infections in the respiratory tract. Scand J Infect Dis Suppl. 1986;49:135–9.
Cometta A, Baumgartner JD, Lew D, et al. Prospective randomized comparison of imipenem monotherapy with imipenem plus netilmicin for treatment of severe infections in nonneutropenic patients. Antimicrob Agents Chemother. 1994;38(6):1309–13. https://doi.org/10.1128/AAC.38.6.1309.
Bliziotis IA, Samonis G, Vardakas KZ, Chrysanthopoulou S, Falagas ME. Effect of aminoglycoside and beta-lactam combination therapy versus beta-lactam monotherapy on the emergence of antimicrobial resistance: a meta-analysis of randomized, controlled trials. Clin Infect Dis. 2005;41(2):149–58. https://doi.org/10.1086/430912.
Leng Y, Chen C, Zhang Y, Luo C, Liu B. Ability of serum procalcitonin to distinguish focus of infection and pathogen types in patients with bloodstream infection. Ann Transl Med. 2019;7(7):135. https://doi.org/10.21037/atm.2019.03.24.
Sakran JV, Michetti CP, Sheridan MJ, et al. The utility of procalcitonin in critically ill trauma patients. J Trauma Acute Care Surg. 2012;73(2):413–8. https://doi.org/10.1097/TA.0b013e31825ff5b7.
Deng S, Zhu H, Wang K, Cao T. Procalcitonin as a marker of sepsis and outcome in patients with neurotrauma: an observation study. BMC Anesthesiol. 2013;13(1):48. https://doi.org/10.1186/1471-2253-13-48.
Zycinska K, Wardyn KA, Zielonka TM, Tyszko P, Straburzynski M. Procalcitonin as an indicator of systemic response to infection in active pulmonary Wegener’s granulomacytosis. J Physiol Pharmacol. 2008;59(Suppl 6):839–44.
Zhang N, Sun J, Ji C, Bao X, Yuan C. Predicting bacterial infection risk in patients with ANCA-associated vasculitis in southwest China: development of a new nomogram. Clin Rheumatol. 2022;41(11):3451–60. https://doi.org/10.1007/s10067-022-06314-9.
Liu C, Chen K, Wu Y, et al. Epidemiological and genetic characteristics of clinical carbapenem-resistant Acinetobacter baumannii strains collected countrywide from hospital intensive care units (ICUs) in China. Emerg Microbes Infect. 2022;11(1):1730–41. https://doi.org/10.1080/22221751.2022.2093134.
Zhang L, Wang Z, Xu F, et al. Effects of Stress Hyperglycemia on Short-Term Prognosis of Patients Without Diabetes Mellitus in Coronary Care Unit. Front Cardiovasc Med. 2021;8: 683932. https://doi.org/10.3389/fcvm.2021.683932.
Zhou S, Zeng Z, Wei H, et al. Early combination of albumin with crystalloids administration might be beneficial for the survival of septic patients: a retrospective analysis from MIMIC-IV database. Ann Intensive Care. 2021;11(1):42. https://doi.org/10.1186/s13613-021-00830-8.
He T, Li J, Wang P, et al. Artificial intelligence predictive system of individual survival rate for lung adenocarcinoma. Comput Struct Biotechnol J. 2022;20:2352–9. https://doi.org/10.1016/j.csbj.2022.05.005.
Fijačko N, Masterson Creber R, Gosak L, et al. A Review of Mortality Risk Prediction Models in Smartphone Applications. J Med Syst. 2021;45(12):107. https://doi.org/10.1007/s10916-021-01776-x.
Wells S, Furness S, Rafter N, et al. Integrated electronic decision support increases cardiovascular disease risk assessment four fold in routine primary care practice. Eur J Cardiovasc Prev Rehabil. 2008;15(2):173–8. https://doi.org/10.1097/HJR.0b013e3282f13af4.
Acknowledgements
The authors acknowledge and thank all the staff of the laboratory and all the clinicians and staff of the neurosurgical critical care unit.
Funding
This research was supported by the Analysis and Knowledge Services of Yangzhou University (YBK202202), the National Natural Science Foundation of China (No. 82172603), the Natural Science Foundation of Jiangsu Province (BK20221280), the Special Fund for Social Key Research and Development Plan of Yangzhou City (YZ2022097), the Chinese Postdoctoral Science Foundation (2022M711426), Jiangsu Provincial Science and Technology Programme Special Funds (Key R&D Programme for Social Development) (Grant no. BE2022775), the Jiangsu Provincial Health Commission New Technology Introduction and Evaluation Project (M2022044).
Author information
Contributions
YPL and GYL conceived and designed the study. XRG analyzed the data and wrote the first draft. TS, JYZ, YTL, QPZ, JLD, JC, KY, QM, XGL, HLY took charge of the collection of all data. GYL gave important suggestions on improving the quality of the analysis and monitored the study’s progress as well as revised the manuscript. All authors contributed to the data collection, checking, and processing. All authors reviewed the final version of the manuscript. All authors read and approved the final manuscript.