Abstract
Background
Immunosuppressive properties grant mesenchymal stromal cells (MSCs) promising potential for treating autoimmune diseases. As autologous MSCs suffer from limited availability, the readily available allogeneic MSCs isolated from menstrual blood (MB-MSCs) donated by young, healthy individuals offer great potential. Here, we evaluate the therapeutic potential of MB-MSCs as ready-to-use allo-MSCs in multiple sclerosis, an autoimmune disease developed by the activation of myelin sheath-reactive Th1 and Th17 cells, by application in its animal model experimental autoimmune encephalomyelitis (EAE).
Methods
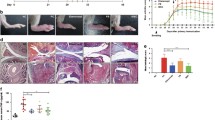
We assessed the therapeutic effect of MB-MSCs transplanted via either intravenous (i.v.) or intraperitoneal (i.p.) route in EAE in comparison with umbilical cord-derived MSCs (UC-MSCs). We used histology to assess myelin sheath integrity and infiltrated immune cells in CNS and flow cytometry to evaluate EAE-associated inflammatory T cells and antigen-presenting cells in lymphoid organs.
Results
We observed disease-ameliorating effects of MB-MSCs when transplanted at various stages of EAE (day − 1, 6, 10, and 19), via either i.v. or i.p. route, with a potency comparable to UC-MSCs. We observed reduced Th1 and Th17 cell responses in mice that had received MB-MSCs via either i.v. or i.p. injection. The repressed Th1 and Th17 cell responses were associated with a reduced frequency of plasmacytoid dendritic cells (pDCs) and a suppressed co-stimulatory capacity of pDCs, cDCs, and B cells.
Conclusions
Our data demonstrate that the readily available MB-MSCs significantly reduced the disease severity of EAE upon transplantation. Thus, they have the potential to be developed as ready-to-use allo-MSCs in MS-related inflammation.
Graphical abstract

Similar content being viewed by others
Background
Mesenchymal stromal cells (MSCs) are nonhematopoietic progenitor cells primarily located in umbilical cord, bone marrow, and adipose tissue. MSCs have the potential to differentiate into multiple lineages, including chondrocytes, adipocytes, as well as osteoblasts [1, 2], and are thus considered a promising tool for cell-based regenerative therapy [3]. Data from preclinical studies demonstrate that an intrinsic immunosuppressive capacity of MSCs constitutes a major part of their therapeutic effects [4, 5]. In addition, their low immunogenicity, due to a modest expression of MHC-I and a complete lack of MHC-II [6, 7] and co-stimulatory molecules, helps them to avoid immune surveillance [8].
While MSCs can be isolated from patients and re-applied as autologous MSCs to avoid immune rejection, certain types of diseases restrict patients from supplying MSCs by themselves. For example, myelofibrosis impairs the quality of bone marrow-derived MSCs (BM-MSCs) [9], and systemic diseases, such as diabetes, RA, and SLE alter the intrinsic properties of MSCs [10,11,12]. Patient age also heavily impacts the availability and functionality of MSCs, as it is generally difficult to procure and extract MSCs from infants, while MSCs from the elderly display decreased biological activity and hence deficits in differentiation and immunoregulation potential [13,14,15,16]. In addition, acute diseases such as stroke and myocardial infarction do not allow enough time to extract and expand autologous MSCs and instead need ready-to-use products. Allo-MSCs from young healthy donors are a plausible approach to overcome these difficulties. Indeed, transplantation of human BM-MSCs has been approved for the management of refractory acute GVHD in children unresponsive to systemic steroid therapies. Currently, they are also tested in clinical trials for Crohn's disease [17], GVHD [18, 19], epidermolysis bullosa [20], COVID-19 [21], and for the repair of heart tissue following myocardial infarction [22]. So far, initial results showed that they are well tolerated.
Menstrual blood, monthly shed by women above twelve to fifteen years of age, contains self-renewing stromal cells. In 2007, Meng et al. first isolated MB-MSCs and confirmed their MSC properties, including MSC surface marker expression, self-renewal, and trilineage differentiation potential [23]. MB-MSCs can be collected regularly and non-invasively, providing important potential for biobanking. With regard to stromal cell biological properties, MB-MSCs are comparable to other MSCs with a high proliferation rate [23]. Recent work from the group of Li and her collaboration partners has essentially proven the safety and efficiency of MB-MSCs as allo-MSCs in treating COVID-19 patients [24]. To date, no study has evaluated the therapeutic effects of MB-MSCs in preclinical models of MS.
MS is a neurodegenerative autoimmune disease affecting the central nervous system [25]. It is the most common disabling neurological disorder in young adults and the third largest cause of significant disability for adults between 20 and 40 years [26, 27]. Despite the use of new immunomodulatory agents such as Natalizumab, most patients eventually enter relapsing–remitting phases, accompanied by vicious immunodeficiency complications due to unspecific immune cell depletion or inhibition [28]. Moreover, these therapeutic agents lack the capacity to promote remyelination and therefore the potential for repairing patients´ neurological function [29, 30].
Aiming to overcome these critical drawbacks of current MS therapies, we evaluated the therapeutic effects of MB-MSCs—which hold, among other benefits of MSCs, the advantage of non-invasive and periodical acquisition—in the murine MS model EAE. We first show the disease-ameliorating function of MB-MSCs when transplanted at various stages of EAE and via both intravenous and intraperitoneal routes. Further, we found that the disease-ameliorating effect of MB-MSCs was associated with suppressed inflammatory immune responses in both peripheral lymphoid organs and the CNS, represented by repressed APC activity, lower frequencies of Th1 and Th17 cells, and fewer lymphocytes infiltrating the CNS. Lastly, we show that MB-MSCs had therapeutic effects similar to UC-MSCs. Thus, MB-MSCs have the potential to be developed as a read-to-use allo-MSC therapeutic agent.
Methods
Mice and EAE induction
We used female C57BL/6 mice, 8–12 weeks of age at the start of experiments. Mice were housed in the animal facilities of ** MS [51, 52]. Therapeutic effects of MSCs are more desirable as transplantation could be used as a curative approach. Our data demonstrate that MB-MSCs can suppress EAE when transplanted on day six or ten after EAE induction. Even when transplanted on day 19, MB-MSCs provided a rapid improvement in the recovery phase in this model. Whether this translates into a potential therapeutic effect remains to be tested in detail and could be assessed in non-remitting chronic EAE disease models. Overall, we show that MB-MSCs achieved a similar protective effect as UC-MSCs and thus should be considered a candidate for ready-to-use allo-MSC products.
The administration route determines the microenvironment that MSCs first encounter in recipients and may thus influence their immunosuppressive mechanisms. Intravenous injection is most common due to its convenience; however, MSCs administered via this route are easily trapped in lung capillaries and thus may fail to enter the peripheral immune system [31]. Further, MSC injection via the i.v. route is accompanied by the risk of instant blood-mediated inflammatory reactions that compromise safety and therapeutic efficacy [3, 53, 54]. As an alternative systemic delivery, MB-MSCs can be transplanted via the i.p. route. In this way, the cells are not trapped in the lungs and do not cause hemocompatibility-related issues, while they still generated a comparable protective effect. The main mechanisms of action of MSCs in supporting tissue regeneration and immunomodulation are cell-contact-dependent or -independent mechanisms, mainly mediated via the secretion of trophic and immunomodulatory factors [3, 53, 55]. Hence, the fact that both i.v.- and i.p.-delivered MB-MSCs are therapeutically beneficial provides broader opportunities for MSC administration.
The exact mechanisms by which MB-MSCs mediate their beneficial outcomes have remained ill defined. Our data demonstrate an association of ameliorated disease with suppressed Th1 and Th17 cell responses in the periphery. Interestingly, MB-MSCs delivered i.v. tended to display a stronger suppression of Th1 responses, while MB-MSCs delivered i.p. featured a stronger suppression of Th17 responses. The MB-MSC-induced suppression of CD4 T cell responses was probably mediated by reduced APC activity: The presence of pDC in the spleen was significantly suppressed by both i.v.- and i.p.-delivered MB-MSCs. pDC plays an important role in initiating EAE via promoting the priming of Th1 and Th17 cells [41], so their numeric reduction could explain limited Th1 and Th17 responses in MB-MSC-transplanted mice. CD40 co-stimulation induces IL-12 production and results in the induction of Th1 responses [56, 57]. APCs, especially those of mice that had received MB-MSCs i.v., showed reduced CD40 expression. Thus, this lack of CD40 may provide a molecular explanation of the reduced Th1 response. In addition, surface expression of the co-stimulators CD80, CD86, and OX40L was also reduced on APCs of MB-MSC-transplanted mice.
Conclusions
Human menstrual blood-derived MSCs, which can be isolated non-invasively and could be stored for acute application, ameliorated the disease severity of EAE upon transplantation, regardless of the route or the time of delivery, by suppressing T cell activation in peripheral lymphoid organs and immune cell infiltration into the CNS.
Availability of data and materials
The datasets in this study are available from the corresponding authors upon request.
Abbreviations
- MB-MSC:
-
Menstrual blood-derived mesenchymal stromal cell
- UC-MSC:
-
Umbilical cord-derived mesenchymal stromal cell
- allo-MSC:
-
Allogeneic MSC
- MS:
-
Multiple sclerosis
- EAE:
-
Experimental autoimmune encephalomyelitis
- CNS:
-
Central nervous system
- APC:
-
Antigen-presenting cell
- i.v.:
-
Intravenous injection
- i.p.:
-
Intraperitoneally injection
References
Pittenger MF, Discher DE, Peault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22.
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7.
Moll G, Ankrum JA, Kamhieh-Milz J, Bieback K, Ringden O, Volk HD, et al. Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol Med. 2019;25(2):149–63.
Krampera M, Glennie S, Dyson J, Scott D, Laylor R, Simpson E, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101(9):3722–9.
Siegel G, Schafer R, Dazzi F. The immunosuppressive properties of mesenchymal stem cells. Transplantation. 2009;87(9 Suppl):S45–9.
Wang Y, Tian M, Wang F, Heng BC, Zhou J, Cai Z, et al. Understanding the immunological mechanisms of mesenchymal stem cells in allogeneic transplantation: from the aspect of major histocompatibility complex class I. Stem Cells Dev. 2019;28(17):1141–50.
Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32(3):252–60.
Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal stem cells for regenerative medicine. Cells. 2019;8(8):886.
Schneider RK, Ziegler S, Leisten I, Ferreira MS, Schumacher A, Rath B, et al. Activated fibronectin-secretory phenotype of mesenchymal stromal cells in pre-fibrotic myeloproliferative neoplasms. J Hematol Oncol. 2014;7:92.
van de Vyver M. Intrinsic mesenchymal stem cell dysfunction in diabetes mellitus: implications for autologous cell therapy. Stem Cells Dev. 2017;26(14):1042–53.
Sun Y, Deng W, Geng L, Zhang L, Liu R, Chen W, et al. Mesenchymal stem cells from patients with rheumatoid arthritis display impaired function in inhibiting Th17 cells. J Immunol Res. 2015;2015:284215.
Gao L, Bird AK, Meednu N, Dauenhauer K, Liesveld J, Anolik J, et al. Bone marrow-derived mesenchymal stem cells from patients with systemic lupus erythematosus have a senescence-associated secretory phenotype mediated by a mitochondrial antiviral signaling protein-interferon-beta feedback loop. Arthritis Rheumatol. 2017;69(8):1623–35.
Taguchi T, Borjesson DL, Osmond C, Griffon DJ. Influence of donor’s age on immunomodulatory properties of canine adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 2019;28(23):1562–71.
Wagner DR, Karnik S, Gunderson ZJ, Nielsen JJ, Fennimore A, Promer HJ, et al. Dysfunctional stem and progenitor cells impair fracture healing with age. World J Stem Cells. 2019;11(6):281–96.
Siegel G, Kluba T, Hermanutz-Klein U, Bieback K, Northoff H, Schafer R. Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells. BMC Med. 2013;11:146.
Andrzejewska A, Catar R, Schoon J, Qazi TH, Sass FA, Jacobi D, et al. Multi-parameter analysis of biobanked human bone marrow stromal cells shows little influence for donor age and mild comorbidities on phenotypic and functional properties. Front Immunol. 2019;10:2474.
Carvello M, Lightner A, Yamamoto T, Kotze PG, Spinelli A. Mesenchymal stem cells for perianal crohn’s disease. Cells. 2019;8(7):764.
Zhao L, Chen S, Yang P, Cao H, Li L. The role of mesenchymal stem cells in hematopoietic stem cell transplantation: prevention and treatment of graft-versus-host disease. Stem Cell Res Ther. 2019;10(1):182.
Salmenniemi U, Itala-Remes M, Nystedt J, Putkonen M, Niittyvuopio R, Vettenranta K, et al. Good responses but high TRM in adult patients after MSC therapy for GvHD. Bone Marrow Transplant. 2017;52(4):606–8.
Rashidghamat E, Kadiyirire T, Ayis S, Petrof G, Liu L, Pullabhatla V, et al. Phase I/II open-label trial of intravenous allogeneic mesenchymal stromal cell therapy in adults with recessive dystrophic epidermolysis bullosa. J Am Acad Dermatol. 2020;83(2):447–54.
Shu L, Niu C, Li R, Huang T, Wang Y, Huang M, et al. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11(1):361.
Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J Am Coll Cardiol. 2009;54(24):2277–86.
Meng X, Ichim TE, Zhong J, Rogers A, Yin Z, Jackson J, et al. Endometrial regenerative cells: a novel stem cell population. J Transl Med. 2007;5:57.
Xu X, Jiang W, Chen L, Xu Z, Zhang Q, Zhu M, et al. Evaluation of the safety and efficacy of using human menstrual blood-derived mesenchymal stromal cells in treating severe and critically ill COVID-19 patients: an exploratory clinical trial. Clin Transl Med. 2021;11:e297.
Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–17.
Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A, Vukusic S, et al. Multiple sclerosis. Nat Rev Dis Primers. 2018;4(1):43.
Garg N, Smith TW. An update on immunopathogenesis, diagnosis, and treatment of multiple sclerosis. Brain Behav. 2015;5(9):e00362.
Rommer PS, Milo R, Han MH, Satyanarayan S, Sellner J, Hauer L, et al. Immunological aspects of approved MS therapeutics. Front Immunol. 2019;10:1564.
Kremer D, Kury P, Dutta R. Promoting remyelination in multiple sclerosis: current drugs and future prospects. Mult Scler. 2015;21(5):541–9.
Wooliscroft L, Silbermann E, Cameron M, Bourdette D. Approaches to remyelination therapies in multiple sclerosis. Curr Treat Options Neurol. 2019;21(7):34.
Liu Y, Niu R, Yang F, Yan Y, Liang S, Sun Y, et al. Biological characteristics of human menstrual blood-derived endometrial stem cells. J Cell Mol Med. 2018;22(3):1627–39.
Berglund AK, Fortier LA, Antczak DF, Schnabel LV. Immunoprivileged no more: measuring the immunogenicity of allogeneic adult mesenchymal stem cells. Stem Cell Res Ther. 2017;8(1):288.
Tatone C, Di Emidio G, Vento M, Ciriminna R, Artini PG. Cryopreservation and oxidative stress in reproductive cells. Gynecol Endocrinol. 2010;26(8):563–7.
Bissoyi A, Nayak B, Pramanik K, Sarangi SK. Targeting cryopreservation-induced cell death: a review. Biopreserv Biobank. 2014;12(1):23–34.
Moll G, Geissler S, Catar R, Ignatowicz L, Hoogduijn MJ, Strunk D, et al. Cryopreserved or fresh mesenchymal stromal cells: only a matter of taste or key to unleash the full clinical potential of MSC therapy? Adv Exp Med Biol. 2016;951:77–98.
Gurgul A, Romanek J, Pawlina-Tyszko K, Szmatola T, Opiela J. Evaluation of changes arising in the pig mesenchymal stromal cells transcriptome following cryopreservation and Trichostatin A treatment. PLoS ONE. 2018;13(2):e0192147.
Wang S, Cheng H, Dai G, Wang X, Hua R, Liu X, et al. Umbilical cord mesenchymal stem cell transplantation significantly improves neurological function in patients with sequelae of traumatic brain injury. Brain Res. 2013;1532:76–84.
Lee RH, Pulin AA, Seo MJ, Kota DJ, Ylostalo J, Larson BL, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54–63.
Li J, Chen Y, Chen Z, Huang Y, Yang D, Su Z, et al. Therapeutic effects of human adipose tissue-derived stem cell (hADSC) transplantation on experimental autoimmune encephalomyelitis (EAE) mice. Sci Rep. 2017;7(1):1–10.
Yousefi F, Ebtekar M, Soudi S, Soleimani M, Hashemi SM. In vivo immunomodulatory effects of adipose-derived mesenchymal stem cells conditioned medium in experimental autoimmune encephalomyelitis. Immunol Lett. 2016;172:94–105.
Isaksson M, Ardesjo B, Ronnblom L, Kampe O, Lassmann H, Eloranta ML, et al. Plasmacytoid DC promote priming of autoimmune Th17 cells and EAE. Eur J Immunol. 2009;39(10):2925–35.
Duraes FV, Lippens C, Steinbach K, Dubrot J, Brighouse D, Bendriss-Vermare N, et al. pDC therapy induces recovery from EAE by recruiting endogenous pDC to sites of CNS inflammation. J Autoimmun. 2016;67:8–18.
Li JF, Zhang DJ, Geng T, Chen L, Huang H, Yin HL, et al. The potential of human umbilical cord-derived mesenchymal stem cells as a novel cellular therapy for multiple sclerosis. Cell Transplant. 2014;23(Suppl 1):S113–22.
Riordan NH, Morales I, Fernandez G, Allen N, Fearnot NE, Leckrone ME, et al. Clinical feasibility of umbilical cord tissue-derived mesenchymal stem cells in the treatment of multiple sclerosis. J Transl Med. 2018;16(1):57.
Meng M, Liu Y, Wang W, Wei C, Liu F, Du Z, et al. Umbilical cord mesenchymal stem cell transplantation in the treatment of multiple sclerosis. Am J Transl Res. 2018;10(1):212–23.
Abramowski P, Krasemann S, Ernst T, Lange C, Ittrich H, Schweizer M, et al. Mesenchymal stromal/stem cells do not ameliorate experimental autoimmune encephalomyelitis and are not detectable in the central nervous system of transplanted mice. Stem Cells Dev. 2016;25(15):1134–48.
Chelmicka-Szorc E, Arnason BG. Partial suppression of experimental allergic encephalomyelitis with heparin. Arch Neurol. 1972;27(2):153–8.
Lider O, Baharav E, Mekori YA, Miller T, Naparstek Y, Vlodavsky I, et al. Suppression of experimental autoimmune diseases and prolongation of allograft survival by treatment of animals with low doses of heparins. J Clin Invest. 1989;83(3):752–6.
**n Y, Gao J, Hu R, Li H, Li Q, Han F, et al. Changes of immune parameters of T lymphocytes and macrophages in EAE mice after BM-MSCs transplantation. Immunol Lett. 2020;225:66–73.
Liu S, Wang J, Han R, Meng M, Wang W, Zhao Y, et al. Therapeutic effect of transplanted umbilical cord mesenchymal stem cells in a cynomolgus monkey model of multiple sclerosis. Am J Transl Res. 2019;11(4):2516–31.
Hansen T, Skytthe A, Stenager E, Petersen HC, Bronnum-Hansen H, Kyvik KO. Concordance for multiple sclerosis in Danish twins: an update of a nationwide study. Mult Scler. 2005;11(5):504–10.
Hansen T, Skytthe A, Stenager E, Petersen HC, Kyvik KO, Bronnum-Hansen H. Risk for multiple sclerosis in dizygotic and monozygotic twins. Mult Scler. 2005;11(5):500–3.
Caplan H, Olson SD, Kumar A, George M, Prabhakara KS, Wenzel P, et al. Mesenchymal stromal cell therapeutic delivery: translational challenges to clinical application. Front Immunol. 2019;10:1645.
von Bahr L, Batsis I, Moll G, Hagg M, Szakos A, Sundberg B, et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30(7):1575–8.
Moll G, Hoogduijn MJ, Ankrum JA. Editorial: safety, efficacy and mechanisms of action of mesenchymal stem cell therapies. Front Immunol. 2020;11:243.
Kelsall BL, Stuber E, Neurath M, Strober W. Interleukin-12 production by dendritic cells. The role of CD40-CD40L interactions in Th1 T-cell responses. Ann N Y Acad Sci. 1996;795:116–26.
Hirohata S. Human Th1 responses driven by IL-12 are associated with enhanced expression of CD40 ligand. Clin Exp Immunol. 1999;115(1):78–85.
Acknowledgements
We would like to thank Professor Ciqing Yang for his contribution in immunohistochemical experiments.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the National Natural Science Foundation of China (81671619 and U1804186), Project of Science and Technology Department of Henan Province (212102310822), German Research Foundation (SFB650, grant TP28, and Grants LO 1542/4-1 and LO 1542/5-1), Willy Robert Pitzer Foundation (Osteoarthritis Research Program), and Einstein Center for Regenerative Therapies (EZ-2016-289).
Author information
Authors and Affiliations
Contributions
PS, ML, and JL contributed to project design and further development. YL, HG, PS, TB, XH, YY, YL, LQ, PW, QL, ML, and FY, contributed to the data collection, analysis, and interpretation. PS, ML, TB, YL, and JL contributed to manuscript construction and writing. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the ethical review board of **nxiang Medical University and the Landesamt für Gesundheit und Soziales, Berlin, Germany.
Consent for publication
This paper does not contain data from any individual person, and written informed consent for publication was obtained from all participants.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1
. Kinetics of T cell responses in dLN and spleen, and immune cell infiltration in CNS during the course of EAE. EAE was induced in mice and immune cells were analyzed at various time points thereafter. (a, b) IFNγ- and IL-17-expressing CD4+ T cells in dLN and spleen were determined by FACS after MOG35-55 peptide restimulation. (c) CNS-infiltrating immune cells with high CD45 expression were determined by FACS ex vivo. Pool of two experiments with two mice per time point (n = 4 per time point). Figure S2. MB-MSC transplantation does not affect the frequency of regulatory T cells. EAE was induced in mice and 6 days later, P4 MB-MSCs were transplanted i.v.. Mice that received only PBS served as untreated controls. On day 7, the percentages of Foxp3-expressing cells among CD4 T cells in spleen were determined by FACS. Pool of four experiments with two mice per group per experiment (n = 8). Figure S3. MB-MSCs transplanted either i.v. or i.p. did not affect the accumulation and activation of macrophages, neutrophils, and monocytes. EAE was induced in mice and 6 days later, P4 MB-MSCs were transplanted via i.v. (i.v.) or i.p. (i.p.) route. Mice that received only PBS served as untreated controls. On day 7, the accumulation and activation of macrophages, monocytes, and neutrophils were analyzed in spleen. (a) Representative FACS plots and percentages of macrophages, monocytes, and neutrophils. (b) The expression level of costimulatory molecules on these three types of cells. Pool of four experiments with two mice per group per experiment (n = 8).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, Y., Gao, H., Brunner, T.M. et al. Menstrual blood-derived mesenchymal stromal cells efficiently ameliorate experimental autoimmune encephalomyelitis by inhibiting T cell activation in mice. Stem Cell Res Ther 13, 155 (2022). https://doi.org/10.1186/s13287-022-02838-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13287-022-02838-8