Abstract
Clinical manifestations of COVID-19 patients are dominated by respiratory symptoms, but cardiac complications are commonly observed and associated with increased morbidity and mortality. Underlying pathological mechanisms of cardiac injury are still not entirely elucidated, likely depending on a combination of direct viral damage with an uncontrolled immune activation. Cardiac involvement in these patients ranges from a subtle myocardial injury to cardiogenic shock. Advanced cardiac imaging plays a key role in discriminating the broad spectrum of differential diagnoses. Present article aims to review the value of advanced multimodality imaging in patients with suspected SARS-CoV-2-related cardiovascular involvement and its essential role in risk stratification and tailored treatment strategies. Based on our experience, we also sought to suggest possible diagnostic algorithms for the rationale utilization of advanced imaging tools, such as cardiac CT and CMR, avoiding unnecessary examinations and diagnostic delays.
Similar content being viewed by others
Key points
-
1.
Cardiac involvement is common in COVID-19 patients, leading to a morbidity/mortality increase.
-
2.
Cardiac complication includes, among others, myocarditis, acute coronary syndrome and thromboembolic events.
-
3.
Advanced imaging plays a key role in differential diagnosis of cardiac manifestations.
Introduction
Coronavirus disease 2019 (COVID-19) is a pandemic disease caused by a novel single-stranded enveloped RNA virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the 7th known human coronavirus.
The virus enters cells through the angiotensin-converting enzyme 2 (ACE2) receptor, mostly expressed in lung alveolar cells, cardiac myocytes and vascular endothelial cells.
Clinical manifestations of COVID-19 are dominated by respiratory symptoms, due to the tropism of the SARS-CoV-2 for the lungs, where it causes interstitial pneumonia [1]. The most common severe complications are acute respiratory disease syndrome (ARDS) and systemic inflammatory response syndrome (SIRS), which can lead to multiorgan failure (MOF) and shock.
Both direct and indirect involvement of other organs is common, and, although the underlying pathological mechanism is still under investigation, the cardiovascular system seems to be particularly affected.
Cardiac injury was early recognized among COVID-19 cases in China; in the report from the National Health Commission almost 11.8% of patients without underlying cardiovascular disease had cardiac injury during hospitalization, showed by elevated T-troponin (TnT) levels and/or new onset of ECG/echocardiographic abnormalities [2].
In addition to its prevalence, cardiac injury seems to be significantly associated with fatal outcome.
Retrospective studies among hospitalized COVID-19 patients have reported a more severe respiratory disease in patients with cardiac injury, requiring in greater proportion both noninvasive and invasive mechanical ventilation [3], and markedly higher mortality rate [3, 4].
Several case reports and case series released so far describe cardiovascular manifestations among COVID-19 patients, such as myocarditis [5, 6], acute coronary syndrome (ACS) [7], arrhythmias, pericarditis [8] and venous thromboembolic events.
Along with the understanding of the underlying pathological mechanisms of myocardial injury, a prompt diagnosis becomes essential for risk stratification and to define tailored treatment strategies.
Present article aimed to review the role of advanced multimodality imaging in patients with suspected SARS-CoV-2-related cardiovascular involvement.
Current emerging scientific evidence has been combined with our 8-month clinical work as one of the national referrals for COVID-19 infection. Based on our experience, we also sought to suggest possible diagnostic algorithms for the rationale utilization of advanced imaging tools in this specific clinical setting.
Pathophysiology of myocardial injury
Although underlying mechanism leading to cardiac injury in COVID-19 patients is not entirely understood, several potential pathophysiological pathways have been proposed [9, 10].
Most likely scenario comprehends a multifactorial etiology based on a synergic effect of several mechanism, both direct or indirect, including:
-
the so-called “cytokine storm,” whereby an uncontrolled immune cells activation leads to overproduction of pro-inflammatory cytokines, with disruptive consequences ranging from high fever to vascular malfunction, which can lead to inadequate organ blood supply, resulting in MOF. The increase levels of reactive oxygen species (ROS), caused by the pro-inflammatory state, result in endothelial dysfunction, which plays a pivotal role in the genesis of hypertension, atherosclerosis and other cardiovascular diseases (CVD);
-
direct cytotoxic effects on interstitial cells or macrophages within cardiac tissue, hypothesis supported by viral genome detection within the myocardium in recent autoptic studies [11];
-
potential downregulation of ACE2 expression in the heart, as demonstrated in a murine model of SARS-CoV-2 infection by Oudit et al. [12]. ACE2 seems to provide a protective effect on cardiovascular system, through several mechanism, including anti-inflammation, anti-fibrosis, anti-oxidation, and vasodilation [13]. Therefore, ACE2 under expression in COVID-19 patients is supposed to lead to cardiac dysfunction and progression of atherosclerosis;
-
low oxygen blood levels, detected in this cohort of patients as a consequence of pulmonary disfunction, can cause an insufficient energy intake to cardiomyocyte, increasing anaerobic fermentation. Accordingly, intracellular acidosis and ROS production destroy the cell membrane. Moreover, hypoxia can lead to intracellular influx of calcium ions, contributing apoptosis of cardiomyocytes [14];
-
collateral effects of several drugs widely used in COVID-19 patients, such as antiretroviral therapy, azithromycin and tocilizumab. Indeed, these drugs could lead to electrophysiological alteration as well as be involved in drug–drug interaction with some cardiovascular treatments.
Possible pathogenesis of myocardial involvement is illustrated in Fig. 1.
Hypothetical pathophysiology patterns concurring in cardiovascular involvement in COVID-19. SARS-CoV-2 enters cells via ACE2 receptor in type 2 in pneumocytes, endothelial cells, pericytes and cardiac myocytes, causing direct damage. Systemic inflammation and uncontrolled immune cell activation lead to a ‘cytokine storm’ which can contribute to destabilize atherosclerotic plaques and potentially trigger the onset of myocarditis trough T cells and macrophages infiltrations. Direct viral cardiac injury can provoke the development of arrhythmias, as well as several medications used in COVID-19 patients
From this background emerges clearly that SARS-CoV-2 infection is associated with a wide spectrum of cardiovascular (CV) manifestation, ranging from a subtle myocardial injury to cardiogenic shock.
Overall, pneumonia, older age, pre-existing cardiovascular diseases and greater severity of the disease at presentation lead to an increased risk of CV events [15].
Underlying cardiovascular comorbidities
Similarly to influenza virus infections [16, 17], people affected by SARS-CoV-2 with pre-existing CVD have an increased risk of develo** acute myocardial injury [18], resulting in a poor prognosis. It has been described a more severe and acute systemic response to the infection in these patients, with increased leukocyte count, higher levels of cardiac necrosis biomarkers and greater incidence of ARDS [18]. In particular, patients with underlying CVD and higher levels of TnT showed the worst outcome (mortality 69.44%), as compared to both individuals with increased TnT but without CVD comorbidities (mortality 37.5%) and subjects with previous CVD and normal TnT (mortality 13.3%) [4]. As reported in previous studies, the overall percentage of pre-existing CVD in patients with COVID-19 was 24–48%, and the most frequent comorbidities were hypertension (15–31%), diabetes (7–20%), coronary artery disease (CAD) (3–8%) and other CVD (15%) [19,20,21,22]. The prevalence of these diseases was greater among patients admitted to the intensive care unit (ICU) [10].
As explained before, the genesis of CV involvement in COVID-19 remains debated. For what concerns cardiac injury in patients with CVD, several studies speculated about different etiologies of secondary cardiac involvement. The increase in myocardial oxygen demand during the infection could bring to a cardiac decompensation in patients with pre-existing heart failure [18], while the systemic inflammatory release of cytokines could lead to a major risk for atherosclerotic plaque rupture [21]. Then, the increased coagulation activity, expressed by increased D-dimer levels, could induce thrombosis and ischemia [21]. Furthermore, other studies hypothesized that hypoxia could reduce the oxygen supply to the heart, unmasking a CAD or a microvascular pathology causing myocardial infarction with non-obstructive coronary artery (MINOCA) [23].
Finally, in predisposed patients, hypoxia, together with drugs and systemic inflammation, could lead to arrhythmias.
Myocarditis
Myocarditis is a well-recognized complication of acute viral infections [24], as a wide spectrum of viral genomes has been identified in the endomyocardial specimens of patients with clinical suspicion of myocarditis and parvovirus B-19, adenovirus or influenza infection [25].
Myocarditis has also been reported as a complication of middle east respiratory syndrome (MERS), caused by another severe coronavirus [26].
Several case reports of COVID-19 patients with acute myocardial injury, defined as troponin release, provide evidence of cardiac inflammation [27] with cardiac magnetic resonance (CMR) findings compatible with acute myocarditis [28, 29].
Notably, Esposito et al. [30] reported a series of 8 patients with elevated concentrations of TnT and electrocardiography alterations whom CMR findings fulfilled the 2018 Lake Louise Criteria for the diagnosis of myocarditis. In all cases, CMR showed diffuse intense myocardial edema, increased T1 and T2 map** and also a mild pericardial effusion in 75% of patients.
Worth noticing, all patients had no remarkable previous history of cardiovascular disease.
Even Inciardi et al. [31] reported a case of a 53-year-old woman with no CV history presented to the emergency department with severe fatigue and abnormal ECG findings with elevated levels of markers of myocyte necrosis. After urgent invasive coronary angiography (ICA) was performed with no evidence of obstructive coronary disease, CMR showed marked biventricular myocardial interstitial edema and diffuse transmural late gadolinium enhancement (LGE) with circumferential pericardial effusion, with the final diagnosis of myopericarditis.
Although the gold standard in the diagnosis of myocarditis is endomyocardial biopsy (EMB), through histological, immunological and molecular evidences [32], it is rarely performed in COVID-19 patients for obvious implications related to the complexity of organizing a procedure which is invasive and poses all involved operators at risk of infection. In this setting, CMR represents the ideal noninvasive imaging tool for clinical diagnosis of myocarditis.
CMR patterns have been reported to be heterogeneous, as aforementioned, but in general not different from any other typical form of active inflammation characterized by diffuse myocardial edema. LGE seems to be less-frequently observed in these patients [30], reflecting a limited myocyte necrosis at least in acute phase, and highlighting the key role of the new Lake Louise Criteria in myocarditis diagnosis [33].
We noted similar findings in our experience with a crucial role of map** techniques for the assessment of myocardial inflammation.
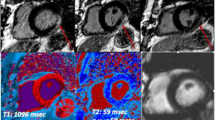
When present LGE has a non-ischemic pattern, as in case showed in Fig. 2, with a predominant location at inferior and inferior-lateral segments [34].
Myocarditis. 51-year-old man hospitalized for COVID‐19 pneumonia and sudden onset of tachyarrhythmias. STIR images revealed no edema (a), although LGE was evident on infero-lateral segments of basal-mid planes with a subepicardial pattern of distribution (b, c orange arrows) and native T1 was increased on LGE + segments (d). T2 map** sequences revealed the presence of edema on infero-lateral segments of mid-ventricular planes (e). ECV confirmed those findings with implemented values on infero-lateral wall (f). Chest CT showed GGO predominantly distributed on inferior lobes with a peripheral distribution (g). STIR short tau inversion recovery, LGE late gadolinium enhancement, ECV extracellular volume, CT computed tomography, GGO ground glass opacity
In their echocardiographic study, Moody et al. have found an independent association between reduced RV systolic dysfunction and all-cause mortality in severe COVID-19 patients with elevated TnT. Besides the effects of thromboembolic disease, authors hypothesized a possible primary RV involvement as a concurrent factor to RV injury [35]. In this clinical setting, a significant contribution could be provided by CMR, which has shown to be the gold standard for the evaluation of RV function together with tissue characterization [36].
Up to date, histopathological confirmation of myocarditis was found in only one patient with CMR findings of reverse Takotsubo syndrome and final diagnosis of lymphocytic myocarditis, with no evidence of SARS-CoV-2 genome within the myocardium [37].
In a cohort study of 39 autopsy cases of patients with COVID-19, Lindner et al. found viral genome in the myocardial tissue in 61.5% of autopsies (n = 24/39), with virus load above 1000 copies in most cases (n = 16/24) [11].
Other autopsy studies [Full size image
Additionally, in COVID-19 era, conventional echocardiography (i.e. not bedside) is potentially underused due to an increased infection risk for healthcare providers during the examination [50].