Abstract
Heavy metals are encountered in nature, and are used in several human endeavors, including in dental fillings. It is well known that the safety of metals depends on their chemical form, as well as the dose and route through which biological systems are exposed to them. Here, we used the Nauphoeta cinerea model to examine the mechanism by which salts of the heavy metals used in dental fillings – silver and mercury – exert their neurotoxicity. Nymphs exposed to heavy metals presented with reduced motor and exploratory abilities as they spent more time immobile, especially in the periphery of a novel object, and covered less distance compared with control nymphs. Exposure to AgNO3 and HgCl2 also exacerbated levels of oxidative stress markers (MDA & ROS) and the neurotransmitter regulators – AChE and MAO, while reducing antioxidant activity markers, both in biochemical (thiol & GST) and RT-qPCR (TRX, GST, SOD, Catalase) examinations, in neural tissues of the cockroach. The observed disruptions in neurolocomotor control, synaptic transmission and redox balance explain how heavy metal salts may predispose organisms to neurological disorders.
Similar content being viewed by others
Introduction
Dental fillings with amalgams are considered safe, despite over a century of deliberations. However, a 2020 US FDA statement posited that certain pre-existing conditions could exacerbate the harmful effects of the mercury vapor that amalgams release [1], and the European Union (EU) parliament has voted to ban dental amalgam use and export from January 2025, while the British Dental Association (BDA) is more inclined toward a phasing-out amalgam fillings [2]. Indeed, continuous and chronic mercury vapor emission from dental amalgam is exacerbated during chewing, tooth brushing and hot fluid intake. Consequently, mercury vapor in absorbed into the lungs, saliva, dental pulp (via the dentinal tubules), as well as the brain and pituitary gland (via the nasal mucosa) [3, 4].
In the elemental state, the constituents of amalgam like silver and mercury are used in dentistry, meteorology, fashion, and finance, amongst other practical applications. On the other hand, organic mercury is the most toxic of the three forms of mercury, and exposure to heavy metal ions via ingestion, inhalation, and dermal contact damages cellular components (cell membrane, DNA, etc.), displaces essential metal ions from enzymes, deform and inactivate enzymes by interacting with amino acid residues on sulfur-containing enzymes, and destabilizes protein structure and function [5]. Subsequently, system-wide disorders, including neurological disorders ensue, and there are records of the development and progression of cancers [6, 7].
Given the increasing awareness that heavy metal toxicity depends on chemical form and concentration, and the understanding that biogeochemical cycling enables heavy metals to be converted from one form to the other in nature and in industrial settings [8, 9], we used the lobster cockroach to study the neurobehavioral, neurotransmitter and redox modifications that follow exposure to the salts of two major components of dental amalgam – silver and mercury. The cockroach has been extensively used to study the neurotoxic outcomes of exposure to organic mercury [10,11,12,13,14] and other xenobiotics [15,16,17], due to the similarity in neuronal signaling from insects to mammals [16, 18, 19]. We therefore explored using the alternate model to understand neurological effects of exposure to heavy metal salts (AgNO3 and HgCl2) in line with the need to replace, reduce, and refine (3Rs) the use of animals in biomedical research.
Materials and methods
Chemicals
Sigma Aldrich Co. (St Louis, Missouri, USA) supplied reduced glutathione and acetylthiocholine iodide. BDH Chemicals Ltd supplied acetic acid, potassium acetate and other chemicals. All chemicals were of analytical grade.
N. cinerea husbandry and experimental protocol
N. cinerea was obtained at the CCNE, Universidade Federal de Santa Maria, Brasil. The cockroaches were maintained at the temperature and humidity of 24 ± 3 °C and 57–75%, respectively, with ad libitum access to water and feed as previously composed [16]. Size matched nymphs were randomly distributed into four groups of 20 nymphs each, including Control (basal diet), basal diet + 272 mg/g HgCl2, basal diet + 85 mg/g AgNO3, and basal diet + 272 mg/g HgCl2 + 85 mg/g AgNO3 and monitored for 7 days. The concentration and duration of exposure were based on the ratio of Hg: Ag in dental amalgam, and the result of pilot studies of feed intake (Table S1) and survival (Fig S1 & S2). Following the exposure period, neurolocomotor assessment was carried out, and nymph heads were excised while on ice, weighed, homogenized (100 mg head: 1 ml 0.1 M Phosphate buffer, pH 7.4 or 100 mg head: 1 ml TRIzol™) and centrifuged (2500 g x 10 min x 4 min) to produce supernatants for biochemical and PCR assays.
Neurobehavioral, neurotransmitter and redox activity assessment
Neurolocomotor activity was filmed for 8 min in a novel environment (white plastic box; 19 × 12.5 × 5 cm) using a webcam mounted over the setup. Data from the video files were analyzed using the ANY-maze 6.0, Steolting, CO, USA video-tracking software [20].
Lipid peroxidation was estimated as described by Ohkawa et al. [21, 22]. 50 µL tissue homogenate was mixed with SDS (150 µL, 8.1%), 20% acetc acid in hydrogen chloride (250 µL, p.H 3.4) and TBA (250 µL, 0.6%). Th tissue homogenate was replaced with distilled water for the blanks. The setup was incubated (1 h x 95 oC) and the product was read at 532 nm. Results were presented as µmol/mg protein.
H2O2 was used as an estimate of ROS (reactive oxygen species) levels as described by Hayashi et al. [23, 24]. Tissue homogenate was incubated at 37 oC in sodium acetate buffer (57 mM, pH 4.8) for 5 min, before the addition of n-n-diethyl-para-phenylenediamine (2.5 mg/Ml) and ferrous sulphate solution (1.8 µM). The absorbance of the resulting product was read at 505 nm and compared against a H2O2 standard calibration curve. Results were expressed as Unit/mg protein.
Total thiol content was estimated by mixing the tissue homogenate (20 µL) with 5,5’-dithiobis-(2-nitrobenzoic acid) (0.5 mM), and potassium phosphate buffer (85 mM, pH 7.4) [25, 26]. Blanks were created without DTNB, and all reaction volumes were left at room temperature for 30 min before the product was read at 412 nm. Results were presented as mmol/mg protein.
Habig and Jakoby’s method of using 1-chloro-2,4-dinitrobenzene (CDNB) as a substrate for estimating glutathione-S-transferase activity was utilized [27, 28]. 100 µL tissue homogenate was mixed with ethylenediaminetetraacetic acid (1 mM), chloro-2, 4-dinitrobenzene (0.80 mM), glutathione as substrate (3.20 mM) and potassium phosphate buffer (70 mM, pH 7.0). The setup was left at room temperature for 10 min and read at 340 nm. Results were expressed as unit/mg protein.
The method of Ellman et al. was used to estimate acetylcholinesterase activity [29, 30]. 30 µL tissue homogenate was mixed with phosphate buffer (10 mM, pH 7.4), 5,5-dithio-bis (2-nitrobenzoic) acid (1 mM), and acetylthiocholine iodide (0.8 mM). The product was read at 412 nm and results were presented as mmolAcSch/h/mg protein.
The method of Mcewen et al. was used to estimate monoamine oxidase content [31]. 50 µL tissue homogenate was mixed with potassium phosphate buffer (72 mM, pH 7.4), benzylamine (0. 5 mM), and distilled water (50 µl). The set up was incubated at 25 oC for 30 min before the addition of 10% perchloric acid and centrifugation (1,500 g x 10 min) to get a clear product that was read at 280 nm. Results were presented as mmol/mg protein.
Nauphoeta cinerea primer sequence was designed for RT-qPCR analysis as earlier presented [16, 17]. Primer efficiency was determined from a 5-point pooled sample dilution, and 40 thermal cycles with a single cycle melt curve was used for the PCR. The Medtl™ System program of Gentier48R™ Real Time PCR with Photodiode detector system (**´an TianLong Science and Technology Co. Ltd, China) was used to access the results which were analysed with the 2−ΔΔCT approach [17, 28, 32] and tubulin was used as the normalizer gene. The primer sequences used included: TRX: F – AGTATCCACGCGCCGTATT; R – TGGGGTCTGCTCCTTGTATC, GST: F – GGGACCTCTGAATGACGAAA; R – CATGCCGTCCAAATAATCAA, SOD: F – GTATTCTGGTGGCTGCGAAA; R – TAAACCCAACACAGAGCCTTG, Catalase: F – ACGAGATCCAGCATCTGACC; R – CTCCACGGTTATCCACAGGT, Tubulin: F – TTGCCAGTGATGAGTTGCTC. R – TAGTGGCTCCAGTGCAAGTC.
Statistical analyses
Data were analysed via one-way Analysis of Variance (ANOVA) and Tukey’s multiple comparisons test and expressed as mean ± SD. Graph pad PRISM (V.8.0) was used for analyses and significance was set at p ≤ 0.05.
Results
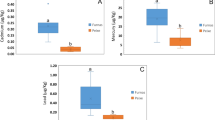
Exposure to mercury and silver salts disrupted motor and exploratory activities in Nauphoeta cinerea as depicted by the track plots (Fig. 1A) and heat maps (Fig. 1B). The total distance travelled (Fig. 1C) and average speed (Fig. 1D) were also reduced, while the total time spent immobile (Fig. 1D) and the time spent in the periphery of the novel environment were increased (Fig. 1E) in the exposed nymphs. Heavy metal salts also increased oxidative stress markers (TBARS: Fig. 2A, ROS: Fig. 2B), reduced antioxidant activity (total thiol: Fig. 2C, GST: Fig. 2D), and increased AChE (Fig. 2E) and MAO (Fig. 2F) activity in the exposed nymphs. The mRNA levels of TRX (Fig. 3A), GST (Fig. 3B), SOD (Fig. 3C) and Catalase (Fig. 3D) were also reduced in nymphs exposed to heavy metal salts.
Exposure to mercury and silver salts disrupts motor and exploratory activities in Nauphoeta cinerea. The ANY-maze (Stoelting CO, USA) video tracking software was used to analyse recordings of an 8-minute trial that was observed in a novel environment. A. Track plot B. heat map C. Total distance travelled D. Average speed E. Total time immobile F. Total time in peripheral zone. Data shown as mean ± SD. * p < 0.05 against control, # p < 0.05 against HgCl2 only, % p < 0.05 against AgNO3 only
Discussion
Heavy metals are ubiquitously present in the environment because of their diverse applications in day-to-day life [33], hence, they are closely monitored to understand their effect on humans, animals, and the environment. Genetic and environmental factors have been shown to determine the degree of heavy metal toxicity, and there are considerations for the dose and route of exposure. The cycling of heavy metals also makes it important to note the chemical form of the metal that an organism has been exposed to [34]. Here, we took advantage of the lobster cockroaches potential for modelling the reaction of chemicals with biological systems to study the salt forms of two heavy metals that are frequently used in dental fillings – silver and mercury.
Silver and mercury salts limited motor and exploratory activities in N. cinerea as the exposed nymphs spent more time immobile, especially in the periphery of the novel object, similar to previously reported heavy metal-induced neurolocomotor deficits [10, 35]. In the same vein, the neurological disorders that ensure from heavy metal exposure have been linked with raised levels of reactive oxygen species (ROS) with a consequent damage to cellular lipids, protein, and DNA [36]. The influence of heavy metals on ROS signaling differs with each chemical specie, but our report of increased oxidative stress markers and reduced antioxidant activity both via biochemical and RT-qPCR investigations in silver and mercury salts is similar to results shown during exposure to organic mercury [10,11,12, 37, 38].
Deranged synaptic transmission and neurotransmitter metabolism also contribute to heavy metal-induced neurological dysfunction [39]. Here, exposure to silver and mercury salts elevated AChE and MAO levels in the neural tissues of cockroaches, reminiscent of increased hippocampal activity of AChE and MAO which potentiates Aβ peptide formation in dementia [40,41,42].
Conclusion and strength of the study
Silver and mercury salts (both individually and in combination) destabilize neuromotor control, synaptic transmission, and neural redox homeostasis, in a manner that might predispose to neurological disorders. This study aptly showcases Nauphoeta cinerea’s potential for modelling chemical reactions in biological systems, in line with the 3R’s approach to toxicity and safety assessment.
Limitation and future perspectives
It is important to understand how individual metals affect biological systems, but exposure to heavy metals in nature is often in several chemical forms and combinations. Hence, new approach methodologies (NAMs) would be required to extrapolate toxicity assessment for the exposure of humans, animals and the environment to xenobiotics.
Data availability
Data is provided within the manuscript and supplementary material. Further information is available from O.C.O upon reasonable request.
Abbreviations
- 3Rs:
-
Replacement, reduction and refinement
- US FDA:
-
United States Food and Drug Administration
- EU:
-
European Union
- RT-qPCR:
-
Quantitative reverse transcription polymerase chain reaction
- AgNO3:
-
Silver nitrate
- HgCl2:
-
Mercury (II) chloride
- MDA:
-
Malondialdehyde
- ROS:
-
Reactive oxygen species
- AChE:
-
Acetylcholinesterase
- MAO:
-
Monoamine oxidase
- TRX:
-
Thioredoxin
- NAMs:
-
New Approach Methodologies
References
FDA Issues Recommendations for Certain High-Risk Groups Regarding Mercury-Containing Dental Amalgam | FDA. (n.d.). https://www.fda.gov/news-events/press-announcements/fda-issues-recommendations-certain-high-risk-groups-regarding-mercury-containing-dental-amalgam (accessed March 22, 2024).
The amalgam ban. What you need to know, (n.d.). https://www.bda.org/news-and-opinion/news/the-amalgam-ban-what-you-need-to-know/ (accessed June 12, 2024).
Gay DD, Cox RD, Reinhardt JW, Chewing Releases Mercury From Fillings. Lancet. 1979;313:985–6. https://doi.org/10.1016/S0140-6736(79)91773-2.
Weiner JA, Nylander M, Berglund F. Does mercury from amalgam restorations constitute a health hazard? Sci Total Environ. 1990;99:1–22. https://doi.org/10.1016/0048-9697(90)90206-A.
Witkowska D, Słowik J, Chilicka K. Heavy Metals and Human Health: possible exposure pathways and the competition for protein binding sites. Molecules. 2021;26. https://doi.org/10.3390/MOLECULES26196060.
Pietrzak S, Wójcik J, Baszuk P, Marciniak W, Wojtyś M, Dębniak T, Cybulski C, Gronwald J, Alchimowicz J, Masojć B, Waloszczyk P, Gajić D, Grodzki T, Jakubowska A, Scott RJ, Lubiński J, Lener MR. Influence of the levels of arsenic, cadmium, mercury and lead on overall survival in lung cancer. Biomolecules. 2021;11:1160. https://doi.org/10.3390/BIOM11081160/S1.
Faik Atroshi, Substances CC. (2018). https://books.google.com/books/about/Cancer_Causing_Substances.html?id=fWiQDwAAQBAJ (accessed March 21, 2024).
Selin NE. Global biogeochemical cycling of mercury: a review. Annu Rev Environ Resour. 2009;34:43–63. https://doi.org/10.1146/ANNUREV.ENVIRON.051308.084314/CITE/REFWORKS.
Huang T, Geng X, Liu X, Liu J, Duan Y, Zhao S, Gupta R. Reduction of HgCl2 to Hg0 in flue gas at high temperature. Part I: influences of oxidative species. Fuel. 2022;324:124417. https://doi.org/10.1016/J.FUEL.2022.124417.
Piccoli BC, Alvim JC, da Silva FD, Nogara PA, Olagoke OC, Aschner M, Oliveira CS, Rocha JBT. High level of methylmercury exposure causes persisted toxicity in Nauphoeta cinerea. Environ Sci Pollut Res. 2019. https://doi.org/10.1007/s11356-019-06989-9.
Afolabi BA, Olagoke OC, Souza DO, Aschner M, Rocha JBT, Segatto ALA. Modified expression of antioxidant genes in lobster cockroach, Nauphoeta cinerea exposed to methylmercury and monosodium glutamate. Chem Biol Interact. 2020;318:108969. https://doi.org/10.1016/J.CBI.2020.108969.
Afolabi BA, Olagoke OC, Souza DO, Aschner M, Rocha JBT, Segatto ALA. Modified expression of antioxidant genes in lobster cockroach, Nauphoeta cinerea exposed to methylmercury and monosodium glutamate. Chem Biol Interact. 2020;318. https://doi.org/10.1016/j.cbi.2020.108969.
Obafemi BA, Adedara IA, Segatto ALA, Souza DO, da Rocha JBT, Olagoke OC. JNK- and Rel-Mediated Regulation of Inflammation and Neurotoxicity in Nauphoeta cinerea Exposed to Methylmercury and Monosodium Glutamate, J Food Biochem 2023 (2023) 1–9. https://doi.org/10.1155/2023/5530698.
Adedara IA, Rosemberg DB, Souza DO, Farombi EO, Aschner M, Rocha JBT. Neuroprotection of luteolin against methylmercury-induced toxicity in lobster cockroach Nauphoeta cinerea. Environ Toxicol Pharmacol. 2016;42:243–51. https://doi.org/10.1016/J.ETAP.2016.02.001.
Afolabi BA, Olagoke OC. High concentration of MSG alters antioxidant defence system in lobster cockroach Nauphoeta cinerea (Blattodea: Blaberidae). BMC Res Notes. 2020;13:217. https://doi.org/10.1186/s13104-020-05056-8.
Olagoke OC, Afolabi BA, Rocha JBT. Streptozotocin induces brain glucose metabolic changes and alters glucose transporter expression in the lobster cockroach ; Nauphoeta cinerea (Blattodea : Blaberidae). Mol Cell Biochem. 2020;1–35. https://doi.org/10.1007/s11010-020-03976-4.
Olagoke OC, Segatto ALA, Afolabi BA, Rocha JBT. Streptozotocin activates inflammation-associated signalling and antioxidant response in the lobster cockroach; Nauphoeta cinerea (Blattodea: Blaberidae). Chem Biol Interact. 2021;345:109563. https://doi.org/10.1016/J.CBI.2021.109563.
Olagoke OC, Segatto ALA, Afolabi BA, Ardisson-Araujo D, Aschner M, Rocha JBT. RPS6 transcriptional modulation in neural tissues of Nauphoeta cinerea during streptozotocin-associated sugar metabolism impairment. Comp Biochem Physiol B Biochem Mol Biol. 2023;263:110785. https://doi.org/10.1016/J.CBPB.2022.110785.
Gaburro J, Nahavandi S, Bhatti A. Insects neural model: potential alternate to mammals for electrophysiological studies, (2017) 119–30. https://doi.org/10.1007/978-981-10-3957-7_6.
Afolabi BA, Adedara IA, Souza DO, Rocha JBT. Dietary co-exposure to methylmercury and monosodium glutamate disrupts cellular and behavioral responses in the lobster cockroach, Nauphoeta cinerea model. Environ Toxicol Pharmacol. 2018;64:70–7. https://doi.org/10.1016/J.ETAP.2018.09.003.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–8. https://doi.org/10.1016/0003-2697(79)90738-3.
Ogunsuyi OB, Oboh G, Oluokun OO, Ademiluyi AO, Ogunruku OO. Gallic acid protects against neurochemical alterations in transgenic Drosophila model of Alzheimer’s disease. Orient Pharm Exp Med. 2019;20:89–98. https://doi.org/10.1007/S13596-019-00393-X/METRICS.
Hayashi I, Morishita Y, Imai K, Nakamura M, Nakachi K, Hayashi T. High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat Research/Genetic Toxicol Environ Mutagen. 2007;631:55–61. https://doi.org/10.1016/J.MRGENTOX.2007.04.006.
Oboh G, Ogunsuyi OB, Ojelade MT, Akomolafe SF. Effect of dietary inclusions of bitter Kola seed on geotactic behavior and oxidative stress markers in Drosophila melanogaster. Food Sci Nutr. 2018;6:2177–87. https://doi.org/10.1002/FSN3.782.
Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205. https://doi.org/10.1016/0003-2697(68)90092-4.
Ogunsuyi OB, Olagoke OC, Afolabi BA, Loreto JS, Ademiluyi AO, Aschner M, Oboh G, Barbosa NV, da Rocha JBT. Effect of Solanum vegetables on memory index, redox status, and expressions of critical neural genes in Drosophila melanogaster model of memory impairment. Metab Brain Dis. 2022;37:729–41. https://doi.org/10.1007/S11011-021-00871-9/METRICS.
Habig W.H. W.B. Jakoby 1981 Assays for differentiation of glutathione S-transferases. Methods Enzymol 77 398–405 http://www.ncbi.nlm.nih.gov/pubmed/7329316 accessed November 1, 2018.
Ogunsuyi OB, Olagoke OC, Afolabi BA, Oboh G, Ijomone OM, Barbosa NV, da Rocha JBT. Dietary inclusions of Solanum vegetables mitigate aluminum-induced redox and inflammation-related neurotoxicity in Drosophila melanogaster model. Nutr Neurosci. 2022;25:2077–91. https://doi.org/10.1080/1028415X.2021.1933331.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. https://doi.org/10.1016/0003-9861(59)90090-6.
Ogunsuyi OB, Aro OP, Oboh G, Olagoke OC. Curcumin improves the ability of donepezil to ameliorate memory impairment in Drosophila melanogaster: involvement of cholinergic and cnc/Nrf2-redox systems. Drug Chem Toxicol. 2023;46:1035–43. https://doi.org/10.1080/01480545.2022.2119995.
Mcewen CM. Human Plasma Monoamine Oxidase I. Purification and identification, the.iournal of biolwic ∼ L Chemistry 240 (n.d.).
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆CT method. Methods. 2001;25:402–8. https://doi.org/10.1006/meth.2001.1262.
Okereafor U, Makhatha M, Mekuto L, Uche-Okereafor N, Sebola T, Mavumengwana V. Toxic metal implications on agricultural soils, plants, animals, aquatic life and Human Health. Int J Environ Res Public Health 2020. 2020;17:2204. https://doi.org/10.3390/IJERPH17072204.
Gochfeld M. Factors influencing susceptibility to metals. Environ Health Perspect. 1997;105:817–22. https://doi.org/10.1289/EHP.97105S4817.
Pimentel-Acosta CA, Ramírez-Salcedo J, Morales-Serna FN, Fajer-ávila EJ, Chávez-Sánchez C, Lara HH, García-Gasca A. Molecular effects of Silver nanoparticles on Monogenean parasites: lessons from Caenorhabditis elegans. Int J Mol Sci 2020. 2020;21:21. https://doi.org/10.3390/IJMS21165889.
Paithankar JG, Saini S, Dwivedi S, Sharma A, Chowdhuri DK. Heavy metal associated health hazards: an interplay of oxidative stress and signal transduction. Chemosphere. 2021;262:128350. https://doi.org/10.1016/J.CHEMOSPHERE.2020.128350.
Nogara P.A., Oliveira C.S., Schmitz G.L., Piquini P.C., Farina M., Aschner M., Rocha J.B.T. Methylmercury’s chemistry: from the environment to the mammalian brain. Biochim et Biophys Acta (BBA) - Gen Subj. 2019. https://doi.org/10.1016/J.BBAGEN.2019.01.006.
Adedara IA, Rosemberg DB, Souza DO, Kamdem JP, Farombi EO, Aschner M, Rocha JBT. Biochemical and behavioral deficits in the lobster cockroach Nauphoeta cinerea model of methylmercury exposure. Toxicol Res (Camb). 2015;4:442–51. https://doi.org/10.1039/C4TX00231H.
Wu Llin, Gong W, Shen SP, Wang ZH, Yao JX, Wang J, Yu J, Gao R, Wu G. Multiple metal exposures and their correlation with monoamine neurotransmitter metabolism in Chinese electroplating workers. Chemosphere. 2017;182:745–52. https://doi.org/10.1016/J.CHEMOSPHERE.2017.04.112.
Ogunsuyi OB, Olasehinde TA, Oboh G. Neuroprotective properties of solanum leaves in transgenic Drosophila melanogaster model of Alzheimer’s disease. Biomarkers. 2022;27:587–98. https://doi.org/10.1080/1354750X.2022.2077446.
Agrawal R, Tyagi E, Shukla R, Nath C. A study of brain insulin receptors, AChE activity and oxidative stress in rat model of ICV STZ induced dementia. Neuropharmacology. 2009;56:779–87. https://doi.org/10.1016/J.NEUROPHARM.2009.01.005.
Li Q, Li X, Tian B, Chen L. Protective effect of pterostilbene in a streptozotocin-induced mouse model of Alzheimer’s disease by targeting monoamine oxidase B. J Appl Toxicol. 2022;42:1777–86. https://doi.org/10.1002/JAT.4355.
Acknowledgements
Not applicable.
Funding
This study is financially supported in part by the 2023 African-German Network of Excellence in Science (AGNES) Junior Research Grant to OBO.
Author information
Authors and Affiliations
Contributions
O.C.O interpreted the resulst and wrote the manuscript, O.B.O performed experiments and prepared figures, F.E.M performed experiments, J.B.R and G.O edited the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Olagoke, O.C., Ogunsuyi, O.B., Mayokun, F.E. et al. Neurobehavioral, neurotransmitter and redox modifications in Nauphoeta cinerea under mixed heavy metal (silver and mercury) exposure. BMC Res Notes 17, 188 (2024). https://doi.org/10.1186/s13104-024-06852-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13104-024-06852-2