Abstract
Background
Antibiotic prophylaxis is recommended during ANCA-associated vasculitis (AAV) induction. We aimed to describe the frequency, persistence, and factors associated with trimethoprim-sulfamethoxazole (TMP-SMX) use in an adult population sample with granulomatosis with polyangiitis (GPA) treated with rituximab (RTX).
Methods
We identified adults with GPA treated with RTX within the Merative™ Marketscan® Research Databases (2011–2020). TMP-SMX prophylaxis was defined as a \(\ge\) 28-day prescription dispensed within a month of starting RTX. We estimated TMP-SMX persistence, allowing prescription refill gaps of 30 days. Multivariable logistic regression and Cox proportional hazards regression assessed the factors associated with baseline TMP-SMX use and persistence, respectively. Covariates included age, sex, calendar year, insurance type, immunosuppressant use, hospitalization, and co-morbidities.
Results
Among 1877 RTX-treated GPA patients, the mean age was 50.9, and 54% were female. A minority (n = 426, 23%) received TMP-SMX with a median persistence of 141 (IQR 83–248) days. In multivariable analyses, prophylaxis was associated with prednisone use in the month prior to RTX (\(\ge\) 20 mg/day vs none, OR 3.96; 95% CI 3.0–5.2; 1–19 mg/day vs none, OR 2.63; 95% CI 1.8–3.8), and methotrexate use (OR 1.48, 95% CI 1.04–2.1), intensive care (OR 1.95; 95% CI 1.4–2.7), and non-intensive care hospitalization (OR 1.56; 95% CI 1.2–2.1) in the 6 months prior to RTX. Female sex (OR 0.63; 95% CI 0.5–0.8) was negatively associated with TMP-SMX use.
Conclusions
TMP-SMX was dispensed to a minority of RTX-treated GPA patients, more often to those on glucocorticoids and with recent hospitalization. Further research is needed to determine the optimal use and duration of TMP-SMX prophylaxis following RTX in AAV.
Similar content being viewed by others
Introduction
The anti-neutrophil cytoplasm antibody (ANCA)-associated vasculitides (AAV) are life-threatening systemic necrotizing small vessel vasculitides [1]. Rituximab (RTX) has increasingly become a first-line induction and maintenance treatment of severe granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA) [2, 3]. However, serious infections, which occur in approximately one-quarter of patients during AAV treatment [4,5,6,7,8,9,10], are a significant complication and may result in death [11, 12]. Strategies to reduce serious infections are therefore a priority.
Low-dose trimethoprim-sulfamethoxazole (TMP-SMX) prophylaxis is recommended during AAV induction to prevent Pneumocystis jirovecii pneumonia (PJP) [13,14,15,16]. Furthermore, an early randomized controlled trial [17] and recent observational studies [18, 19], including a post hoc analysis of the RAVE trial [20], found an association between TMP-SMX use and reduced overall (all-cause) serious infections in AAV. Recently, the American College of Rheumatology [3] and the Canadian Vasculitis Research Network [2] recommended TMP-SMX prophylaxis (or alternatives, in the case of allergy/intolerance) during RTX induction and for at least 6 months following the last RTX dose. The British Society of Rheumatology suggests continuing prophylaxis during RTX maintenance therapy, especially in high-risk patients, such as those with structural lung disease, prolonged glucocorticoid use, and increased age [21].
In the decade leading up to these recommendations, real-world patterns of TMP-SMX prophylaxis in AAV following treatment with RTX are unknown. Our objective was to describe the frequency and persistence of TMP-SMX prophylaxis in patients with granulomatosis with polyangiitis (GPA) treated with RTX in a US population sample and determine factors associated with prophylaxis.
Methods
Data source
We identified patients with GPA within the Merative™ Marketscan® Research Databases, which comprises US administrative health data for beneficiaries of employer-sponsored health insurance and some smaller commercial insurance plans (patients aged < 65), Medicare-eligible retirees with employer-sponsored Medicare supplemental plans (patients aged ≥ 65), and a subsample of Medicaid enrollees from participating state Medicaid programs. In these data, International Classification of Diseases (ICD) diagnostic codes (from billing and hospitalizations), Current Procedural Terminology (CPT) codes, and National Drug Codes (NDC) identify medical claims from physician outpatient visits, hospitalizations, procedures, and prescription drug dispensations. Prior studies have evaluated GPA epidemiology and cost within MarketScan [10, 22, 23].
Ethical approval
This study complies with the Declaration of Helsinki and was approved by the McGill University Faculty of Medicine Institutional Review Board (22-01-037).
Cohort selection
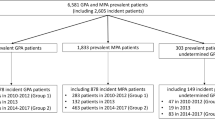
We identified patients with GPA insured between January 1, 2011, and December 31, 2020, according to previously established case definitions [10]. Subjects were required to have at least 1 inpatient claim or 2 outpatient claims at least 30 days apart (but not more than 12 months apart) with a diagnostic code for GPA (i.e., ICD-9 446.4, ICD-10 M31.31, or M31.30) and at least one CPT or NDC code for RTX any time following the first GPA diagnostic code. The combination of ICD coding and specific medication use has a reported sensitivity of up to 94% and a positive predictive value of up to 79% for GPA [24]. We excluded subjects who were aged < 18 at the time of first (index) RTX, if they had any code for RTX prior to the first GPA diagnostic code, or if they had any diagnosis code for eosinophilia (ICD-9 228.3, ICD-10 D72.1) in the year prior to the first GPA code (in order to exclude subjects with eosinophilic granulomatosis with polyangiitis). We did not include MPA, as there is no specific ICD-9 code for this condition. As a sensitivity analysis, we restricted the study population to subjects with continuous eligibility in the database (for medical or drug benefits) for at least 6 months prior to the index RTX procedure code (new-user design, Fig. 1).
Cohort characteristics and exposure definitions
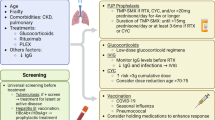
Age, sex, insurance type (commercial, Medicare, Medicaid), and calendar year were determined at the time of index RTX. Because RTX doses are not available in the database, we defined the index RTX treatment as “induction” if it was followed by one or more additional RTX infusions in the following 30 days (to capture regimens of 375 mg/m2 weekly for 4 weeks or 1000 mg every 2 weeks for 2 doses [25]), and defined the index RTX as a “maintenance” treatment if there were no additional infusions in the following 30 days. As patients often start treatment with high-dose glucocorticoids in the weeks prior to receiving RTX induction, we captured prednisone dispensed in the 30 days prior to the index date and categorized the daily dose of the prescription as ≥ 20 mg/day, 1–19 mg/day, or non-use. A 6-month lookback period prior to index RTX also captured recent immunosuppressant use (oral or IV cyclophosphamide, methotrexate, azathioprine), physician visits, hospital admissions (with/without intensive care unit, ICU admission), and serious infections, defined as a hospitalization with a primary diagnosis code for a bacterial or unspecified infection (see Additional file 1: Table S1 for infection ICD codes). We also evaluated patient characteristics using ICD and/or procedure codes for the following disease features and/or co-morbidities anytime prior to index RTX: sinusitis, obstructive lung disease (includes chronic obstructive pulmonary disease, bronchiectasis, and asthma), interstitial lung disease, diabetes, glomerulonephritis, chronic kidney disease, and dialysis.
To identify individuals with potential TMP-SMX allergies or intolerance, we identified ≥ 28-day prescriptions for dapsone and atovaquone (PJP prophylaxis alternatives to TMP-SMX) dispensed in the 6 months prior to and following index RTX.
Outcomes
The primary outcome was baseline TMP-SMX prophylaxis, defined as a ≥ 28-day prescription for TMP-SMX (to exclude intermittent antibiotic prescriptions for acute infection [26]), either dispensed prior to RTX with enough supply to overlap this RTX treatment or dispensed within the 30 days following the index RTX [27].
Analysis
Baseline cohort characteristics were described at the time of index RTX treatment in the observation period, overall and stratified according to TMP-SMX user status. Continuous data were expressed as means (with standard deviation (SD)) or medians (with interquartile range (IQR)). Categorical data were summarized as counts and percentages. We assessed whether TMP-SMX use increased across the calendar year using the Cochran Armitage Trend test.
Univariable logistic regression analyses assessed the association of the following with baseline TMP-SMX use: age, sex, insurance type (commercial/Medicare vs Medicaid), calendar year period (2016–2020 vs 2011–2015), RTX induction (vs maintenance), hospital admission with and without ICU stay (each vs no hospitalization), having at least 1 co-morbid condition (prior ICD codes for lung disease as defined above, diabetes, chronic kidney disease, dialysis), prednisone use in the prior month (≥ 20 mg/day and 1–19 mg/day, each vs none), and use of other immunosuppressants in the prior 6 months. We hypothesized that mean prednisone ≥ 20 mg/day in the month preceding index RTX would be associated with TMP-SMX use, as this is the dose above which experts generally recommend PJP prophylaxis [28]. We expected that TMP-SMX use would be more frequent with RTX induction, as this is a higher risk period for infection. We developed a multivariable model that included relevant potential confounders.
In a pre-specified sensitivity analysis, we restricted the study population to those with 6 months of continuous insurance enrollment prior to index RTX (new user design), in order to limit the inclusion of prevalent RTX users. In the second sensitivity analyses, we liberalized the time window for starting prophylaxis to also include ≥ 28-day prescriptions for TMP-SMX dispensed any time in the 6 months following index RTX (as patients may delay filling prescriptions for a variety of reasons and start prophylaxis later than intended), within the whole study population and in the subgroup with continuous insurance enrollment for the 6 months post-RTX.
Treatment persistence
In the subset of TMP-SMX users with 6 months of continuous insurance enrollment following RTX, we assessed the median duration (in days) of continuous TMP-SMX use (i.e., persistence) from the time of RTX, allowing a maximum refill gap of 30 days between consecutive prescriptions. Kaplan-Meier estimates determined the proportion of subjects remaining on TMP-SMX at 6 months. Cox proportional hazards regression assessed the association between baseline characteristics and time to TMP-SMX discontinuation. Subjects were censored if > 30 days passed with no new prescription dispensed, loss of insurance enrollment, or December 31, 2020, whichever came first. All analyses were performed in SAS (Version 9.4 TS Level 1M6).
Results
Study population
Of 9201 adults meeting GPA ICD code definitions (January 1, 2011, to December 31, 2020), 1877 (20%) received RTX following the first GPA code and met other cohort inclusion criteria (Fig. 1). About half of the cohort was female, and the mean age was 50.9 years at the time of the first (index) RTX infusion (Table 1). The majority (70%) of RTX was induction (according to the study definition). Most (74%) had commercial insurance, while 253 (13%) were covered under Medicare Advantage and 231 (12%) under Medicaid. In the 6 months prior to RTX, 774 (41%) had at least one hospital admission, 317 (17%) required ICU, and 172 (9%) had received dialysis. In the 30 days leading up to RTX, 850 (45%) used prednisone, including 630 (34%) who were dispensed prednisone ≥ 20 mg daily.
TMP-SMX prophylaxis
Baseline TMP-SMX was dispensed to 426 (23%), with the majority (n = 314, 73% of TMP-SMX users) starting prescriptions prior to index RTX and continuing afterwards, and the remainder (n = 112) starting within the month following RTX. TMP-SMX was dispensed to 323/1314 (25%) induction recipients and 247/630 (39%) who were dispensed prednisone ≥ 20 mg daily in the previous month.
Factors associated with baseline TMP-SMX use
In univariable analyses, baseline TMP-SMX use was associated with prednisone ≥ 20 mg/day (vs 0 mg, OR 4.92; 95% CI 3.84–6.33) and prednisone 1–19 mg/day (vs 0 mg, OR 2.86; 95% CI 2.00–4.06) in the month prior to RTX. Induction RTX (vs maintenance), hospitalization with and without ICU stay, prior use of methotrexate, and having a serious infection in the 6 months before RTX were also significantly associated with TMP-SMX use. Although TMP-SMX was more often dispensed in the 2016–2020 period (vs 2011–2015, OR 1.26; 95% CI 1.01–1.57), the p value for the calendar year trend was 0.06. Age (in years, OR 0.99; 95% CI 0.98–0.99) and female sex (OR 0.61; 95% CI 0.49–0.76) were negatively associated with prophylaxis. We did not find any clear associations between co-morbidities (lung disease, chronic kidney disease/dialysis, or diabetes) and TMP-SMX use.
In multivariable analyses, female sex (OR 0.63; 95% CI 0.50–0.80) and age in years (OR 0.99; 95% CI 0.98–0.99) remained negatively associated with TMP-SMX, while prednisone ≥ 20 mg/day (vs 0 mg, OR 3.96; 95% CI 3.04–5.19), prednisone 1–19 mg/day (vs 0 mg, OR 2.63; 95% CI 1.83–3.77), hospitalization with ICU (OR 1.95; 95% CI 1.39–2.73) and without ICU (OR 1.56; 95% CI 1.16–2.09), and methotrexate use (OR 1.48; 95% CI 1.04–2.09) remained associated with TMP-SMX (Table 2).
In the new user design group (with 6 months of continuous insurance enrollment prior to index RTX, N = 919), 281 (31%) were dispensed TMP-SMX at baseline. Multivariable analyses in this subgroup showed similar estimates to the primary analysis (Table 3).
TMP-SMX use in the 6 months following rituximab
Expanding the definition of TMP-SMX use to include ≥ 28-day prescriptions any time in the 6 months following index RTX, we identified 609 TMP-SMX users (183 additional users compared to the primary analysis). Multivariable analysis in the entire cohort (N = 1877; 32% TMP-SMX users) and in the subgroup with 6 months of continuous insurance enrollment following RTX (n = 1308; 38% TMP-SMX users) showed similar results to the primary analyses (Additional file 1: Table S2).
Sex-stratified analyses
To further characterize the negative association between female sex and TMP-SMX use, we stratified clinical characteristics according to sex (Additional file 1: Table S3). Females more frequently had Medicaid (14% vs 10%, difference 4%; 95% CI 2–7%), less frequently had a hospital admission (38% vs 45%, difference 6%; 95% CI 2–10%) and had fewer serious infections (6% vs 11%; difference 4%; 95% CI 2–7%) in the 6 months prior to RTX compared to males. In addition, chronic kidney disease (21% vs 26%, difference 5%; 95% CI 1–9%) and prednisone ≥ 20 mg/day in the prior month (31% vs 36%, difference 5%; 95% CI 1–9%) were less common in females compared to males. When multivariable logistic regression analyses were performed in males and females separately, younger age, rituximab induction (vs maintenance), and prior methotrexate use were significantly associated with TMP-SMX use in females, but we were unable to make definitive conclusions in males (Additional file 1: Table S4).
TMP-SMX persistence
Among 389 TMP-SMX users with \(\ge\) 1 new prescription following index RTX, the median persistence was 141 (Interquartile range 83, 248) days, with 163 (42%) continuing for 6 months or more. In univariable Cox proportional hazards regression analyses, both prednisone ≥ 20 mg/day in the month prior to RTX (HR 1.25; 95% CI 0.98–1.58) and hospitalization in the 6 months prior to RTX (HR 1.24; 95% CI 0.98–1.57) were potentially associated with TMP-SMX persistence (Additional file 1: Table S5).
TMP-SMX prophylaxis alternatives
In the 6 months prior to receiving RTX, 38 (2%) had received at least one prescription for dapsone and 43 (2%) received atovaquone. Similarly, in the 6 months following RTX administration, 41 (2%) received a prescription for dapsone and 48 (3%) received atovaquone.
Discussion
Rituximab is an important therapy for severe GPA, the most common form of AAV in North America [23, 29]. Recent practice guidelines conditionally recommended prophylaxis with TMP-SMX (or alternative) during RTX treatment in AAV (regardless of glucocorticoid dose), acknowledging limited data [2, 3, 21, 30], but little is known about prophylaxis patterns in the real world prior to these recommendations. Based on this large population sample, we estimate that 23–38% of RTX-treated patients with GPA are dispensed TMP-SMX prophylaxis at the time of RTX treatment, depending on the cohort and prophylaxis definitions used. Among TMP-SMX users, the median persistence was nearly 5 months, with 42% continuing prophylaxis for at least 6 months following index RTX.
Therapeutic trials in AAV often leave decisions on antibiotic prophylaxis to local practice [31,32,33] and rarely report the use of prophylaxis with the study results. In the RAVE trial (which compared RTX to cyclophosphamide for GPA and MPA induction) [34], where the majority of subjects received TMP-SMX prophylaxis (as intended in the protocol), the use of TMP-SMX was associated with reduced serious infections [20]. While studies have not assessed the effects of TMP-SMX prophylaxis during RTX maintenance alone, one retrospective study that included patients with repeated RTX infusions over time (mean cumulative RTX 4.75 g with 22 months follow-up) found that TMP-SMX was associated with reduced time to serious infection [18]. In this European tertiary care cohort, 38% of participants were taking TMP-SMX, which is slightly higher than our overall study estimate, but on par with the new user design subgroup (31%). In contrast, at an American tertiary care center, two-thirds of GPA patients (with various treatments) and 68% of RTX users overall (including other systemic rheumatic diseases) were prescribed some form of PJP prophylaxis [26]. However, this study captured intended TMP-SMX prescriptions as recorded in electronic health records, which may be higher than TMP-SMX actually dispensed to patients (as measured in our study). In our study, patients recently taking high-dose prednisone (\(\ge\) 20 mg/day) were especially likely to receive TMP-SMX (39%), which likely reflects an increased perceived risk of PJP and/or other infections in this group. Furthermore, both prednisone use and prior hospitalization were associated with prophylaxis and potentially with TMP-SMX persistence. Higher acuity healthcare encounters and/or high disease activity might increase contact with tertiary care specialists, which could in turn provide more opportunities to prescribe and maintain prophylaxis.
Unexpectedly, females were less likely to be dispensed TMP-SMX. Prior studies did not find that antibiotic prophylaxis differed according to patient sex in AAV [19] or in other systemic diseases treated with immunosuppressants [26, 35] or RTX [27, 36]. In a Japanese prospective inception cohort, the female sex was protective against serious infections in AAV (adjusted HR 0.47 [95% CI 0.25,0.89]) [6], and we observed a small but significant difference in serious infections (in the 6 months prior to RTX) between females (6%) and males (11%) in our cohort. Conversely, the incidence of sulfa antibiotic allergy and antibiotic-associated adverse events might be higher in females [37, 38]. Thus, providers may prescribe TMP-SMX less to female patients due to perceived lower infection risk or concern for adverse events. Interestingly, in our sex-stratified analyses, younger age, RTX induction (vs maintenance), and prior methotrexate use were associated with TMP-SMX in females but not in males. Older females may be less likely to be dispensed TMP-SMX due to known allergies or intolerances identified earlier in life. However, we did not see a difference in the use of atovaquone or dapsone (which might indicate a known TMP-SMX allergy) according to sex. While our results should be interpreted with caution, especially as we lacked granular data to determine whether the omission of TMP-SMX prophylaxis was clinically justified, prior studies have observed sex disparities in the use of preventative treatments in other conditions such as diabetes [39] and peripheral artery disease [40]. The relationship between biological sex (and gender, which we were unable to evaluate) and AAV treatment and prophylaxis choices will require further study.
This large population-based cohort was similar in terms of demographics to other AAV cohorts in the USA [29, 41] and included subjects from different healthcare payors, which adds generalizability of our findings. Nevertheless, our study has limitations. GPA carries a high rate of hospitalization (> 40% of our cohort were hospitalized in the 6 months prior to RTX), but medications administered in the hospital are not captured in MarketScan. Thus, if RTX was only administered in the hospital, a patient may have been inadvertently excluded from the cohort. The lack of data on inpatient medications may have also led to incomplete ascertainment of prednisone exposure among hospitalized patients, potentially explaining the low measured prevalence of prednisone use in our cohort in the month prior to index RTX (34% taking \(\ge\) 20 mg/day). Furthermore, the RTX dose was not available in the database, and our induction definition (≥ 2 RTX infusions within 30 days) may have misclassified maintenance RTX as induction if patients received 2 doses of 500 mg (rather than a single maintenance dose), or misclassified RTX induction as maintenance if only one of the infusions was administered as an outpatient (and therefore captured). However, sensitivity analysis with a new user design (which might enrich the population with induction recipients) found similar estimates. Our study is unique to differentiate RTX induction and maintenance therapy within administrative health data, which is an important consideration for future pharmacoepidemiologic studies in AAV. Although disease activity/severity and kidney function (which could influence TMP-SMX prescribing) are not directly measurable in the database, we included proxies of these measures in our analyses, including prior ICU admission, CYC use, CKD, and dialysis. We only measured TMP-SMX dispensed to the patient, which may underestimate providers’ intended prescriptions. Finally, while we did not differentiate prophylactic from therapeutic doses of TMP-SMX (i.e., double strength twice daily), we expect the minority of patients to be prescribed long-term therapeutic dose TMP-SMX in the last decade, as it is no longer a preferred disease-modifying agent [3].
Conclusions
In conclusion, TMP-SMX was dispensed to less than one-third of US patients with GPA receiving RTX between 2011 and 2020. In our cohort, recent prednisone use and prior hospitalization were associated with TMP-SMX use, while females were less likely to receive prophylaxis. Antibiotic prophylaxis during RTX treatment in AAV is expected to increase following recent recommendations [2, 3, 21, 30]. Further work is needed to determine the association of TMP-SMX use with infectious outcomes in this population, in order to strengthen the evidence on optimal use of TMP-SMX during RTX treatment in AAV.
Availability of data and materials
The data underlying this article are available from Merative™ Marketscan®, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Aggregate data will be shared upon request to the corresponding author with permission from Merative™ Marketscan®.
Abbreviations
- RTX:
-
Rituximab
- AAV:
-
Anti-neutrophil cytoplasm antibody-associated vasculitis
- GPA:
-
Granulomatosis with polyangiitis
- MPA:
-
Microscopic polyangiitis
- TMP-SMX:
-
Trimethoprim-sulfamethoxazole
- CYC:
-
Cyclophosphamide
- MTX:
-
Methotrexate
- ICU:
-
Intensive care unit
- PJP:
-
Pneumocystis jirovecii pneumonia
- US:
-
United States
- ICD:
-
International Classification of Diseases (ICD)
- CPT:
-
Current Procedural Terminology
- NDC:
-
National Drug Code
References
Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L, Hagen EC, et al. 2012 revised International Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65(1):1–11.
Mendel A, Ennis D, Go E, Bakowsky V, Baldwin C, Benseler SM, Cabral DA, Carette S, Clements-Baker M, Clifford AH, et al. CanVasc consensus recommendations for the management of antineutrophil cytoplasm antibody-associated vasculitis: 2020 update. J Rheumatol. 2021;48(4):555–66.
Chung SA, Langford CA, Maz M, Abril A, Gorelik M, Guyatt G, Archer AM, Conn DL, Full KA, Grayson PC, et al. 2021 American College of Rheumatology/Vasculitis Foundation guideline for the management of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 2021;73(8):1366–83.
Pearce FA, McGrath C, Sandhu R, Packham J, Watts RA, Rhodes B, Al-Jayyousi R, Harper L, Obrenovic K, Lanyon P. Outcomes and compliance with standards of care in anti-neutrophil cytoplasmic antibody-associated vasculitis-insights from a large multiregion audit. Rheumatol Adv Pract. 2018;2(2):rky025.
Yoo J, Jung SM, Song JJ, Park YB, Lee SW. Birmingham vasculitis activity and chest manifestation at diagnosis can predict hospitalised infection in ANCA-associated vasculitis. Clin Rheumatol. 2018;37(8):2133–41.
Watanabe-Imai K, Harigai M, Sada KE, Yamamura M, Fujii T, Dobashi H, Amano K, Ito S, Homma S, Kumagai S, et al. Clinical characteristics of and risk factors for serious infection in Japanese patients within six months of remission induction therapy for antineutrophil cytoplasmic antibody-associated vasculitis registered in a nationwide, prospective, inception cohort study. Mod Rheumatol. 2017;27(4):646–51.
Alberici F, Smith RM, Jones RB, Roberts DM, Willcocks LC, Chaudhry A, Smith KG, Jayne DR. Long-term follow-up of patients who received repeat-dose rituximab as maintenance therapy for ANCA-associated vasculitis. Rheumatology (Oxford). 2015;54(7):1153–60.
Yang L, **e H, Liu Z, Chen Y, Wang J, Zhang H, Ge Y, Hu W. Risk factors for infectious complications of ANCA-associated vasculitis: a cohort study. BMC Nephrol. 2018;19(1):138.
Jones RB, Hiemstra TF, Ballarin J, Blockmans DE, Brogan P, Bruchfeld A, Cid MC, Dahlsveen K, de Zoysa J, Espigol-Frigole G, et al. Mycophenolate mofetil versus cyclophosphamide for remission induction in ANCA-associated vasculitis: a randomised, non-inferiority trial. Ann Rheum Dis. 2019;78(3):399–405.
Panupattanapong S, Stwalley DL, White AJ, Olsen MA, French AR, Hartman ME. Epidemiology and outcomes of granulomatosis with polyangiitis in pediatric and working-age adult populations in the United States: analysis of a large national claims database. Arthritis Rheumatol. 2018;70(12):2067–76.
Titeca-Beauport D, Francois A, Lobbedez T, Guerrot D, Launay D, Vrigneaud L, Daroux M, Lebas C, Bienvenu B, Hachulla E, et al. Early predictors of one-year mortality in patients over 65 presenting with ANCA-associated renal vasculitis: a retrospective, multicentre study. BMC Nephrol. 2018;19(1):317.
Singh JA. Infection versus cardiovascular disease as leading causes of hospitalisations and associated mortality in vasculitis in the U.S.: a national study. Clin Exp Rheumatol. 2021;39 Suppl 129(2):56–61.
Green H, Paul M, Vidal L, Leibovici L. Prophylaxis of Pneumocystis pneumonia in immunocompromised non-HIV-infected patients: systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc. 2007;82(9):1052–9.
Lapraik C, Watts R, Bacon P, Carruthers D, Chakravarty K, D’Cruz D, Guillevin L, Harper L, Jayne D, Luqmani R, et al. BSR and BHPR guidelines for the management of adults with ANCA associated vasculitis. Rheumatology (Oxford). 2007;46(10):1615–6.
Charles P, Bienvenu B, Bonnotte B, Gobert P, Godmer P, Hachulla E, Hamidou M, Harle JR, Karras A, Lega JC, et al. Rituximab: recommendations of the French Vasculitis Study Group (FVSG) for induction and maintenance treatments of adult, antineutrophil cytoplasm antibody-associated necrotizing vasculitides. Presse Med. 2013;42(10):1317–30.
Mukhtyar C, Guillevin L, Cid MC, Dasgupta B, de Groot K, Gross W, Hauser T, Hellmich B, Jayne D, Kallenberg CG, et al. EULAR recommendations for the management of primary small and medium vessel vasculitis. Ann Rheum Dis. 2009;68(3):310–7.
Stegeman CA, Tervaert JW, de Jong PE, Kallenberg CG. Trimethoprim-sulfamethoxazole (co-trimoxazole) for the prevention of relapses of Wegener’s granulomatosis. Dutch Co-Trimoxazole Wegener Study Group. N Engl J Med. 1996;335(1):16–20.
Kronbichler A, Kerschbaum J, Gopaluni S, Tieu J, Alberici F, Jones RB, Smith RM, Jayne DRW. Trimethoprim-sulfamethoxazole prophylaxis prevents severe/life-threatening infections following rituximab in antineutrophil cytoplasm antibody-associated vasculitis. Ann Rheum Dis. 2018;77(10):1440–7.
Waki D, Nishimura K, Yoshida T, Tanaka N, Mizukawa K, Fukushima M, Iri O, Anegawa M, Murabe H, Yokota T. Protective effect of different doses of trimethoprim-sulfamethoxazole prophylaxis for early severe infections among patients with antineutrophil cytoplasmic autoantibody-associated vasculitis. Clin Exp Rheumatol. 2021;39 Suppl 129(2):142–8.
Odler B, Riedl R, Gauckler P, Shin JI, Leierer J, Merkel PA, St Clair W, Fervenza F, Geetha D, Monach P, et al. Risk factors for serious infections in ANCA-associated vasculitis. Ann Rheum Dis. 2023;82(5):681–7.
Tieu J, Smith R, Basu N, Brogan P, D’Cruz D, Dhaun N, Flossmann O, Harper L, Jones RB, Lanyon PC, et al. Rituximab for maintenance of remission in ANCA-associated vasculitis: expert consensus guidelines. Rheumatology (Oxford). 2020;59(4):e24–32.
Kong AM, Kim G, Michalska M, Best JH. Costs of disease relapses among individuals with granulomatosis with polyangiitis or microscopic polyangiitis in the United States. Rheumatol Ther. 2018;5(1):159–70.
Raimundo K, Farr AM, Kim G, Duna G. Clinical and economic burden of antineutrophil cytoplasmic antibody-associated vasculitis in the United States. J Rheumatol. 2015;42(12):2383–91.
Sreih AG, Annapureddy N, Springer J, Casey G, Byram K, Cruz A, Estephan M, Frangiosa V, George MD, Liu M, et al. Development and validation of case-finding algorithms for the identification of patients with anti-neutrophil cytoplasmic antibody-associated vasculitis in large healthcare administrative databases. Pharmacoepidemiol Drug Saf. 2016;25(12):1368–74.
Benard V, Farhat C, Zarandi-Nowroozi M, Durand M, Charles P, Puechal X, Guillevin L, Pagnoux C, Makhzoum JP. Comparison of two rituximab induction regimens for antineutrophil cytoplasm antibody-associated vasculitis: systematic review and meta-analysis. ACR Open Rheumatol. 2021;3(7):484–94.
Schmajuk G, Jafri K, Evans M, Shiboski S, Gianfrancesco M, Izadi Z, Patterson SL, Aggarwal I, Sarkar U, Dudley RA, et al. Pneumocystis jirovecii pneumonia (PJP) prophylaxis patterns among patients with rheumatic diseases receiving high-risk immunosuppressant drugs. Semin Arthritis Rheum. 2019;48(6):1087–92.
Park JW, Curtis JR, Jun KI, Kim TM, Heo DS, Ha J, Suh KS, Lee KW, Lee H, Yang J, et al. Primary prophylaxis for Pneumocystis jirovecii pneumonia in patients receiving rituximab. Chest. 2022;161(5):1201–10.
Shafran DM, Bunce PE, Gold WL. Reducing the risk of infection in a 74-year-old man who is to receive prednisone. CMAJ. 2014;186(16):1239–40.
Wallace ZS, Yun H, Curtis JR, Chen L, Stone JH, Choi HK. ANCA-associated vasculitis management in the United States: data from the Rheumatology Informatics System for Effectiveness (RISE) Registry. J Rheumatol. 2021;48(7):1060–4.
Hellmich B, Sanchez-Alamo B, Schirmer JH, Berti A, Blockmans D, Cid MC, Holle JU, Hollinger N, Karadag O, Kronbichler A, et al. EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update. Ann Rheum Dis. 2023. https://doi.org/10.1136/ard-2022-223764.
Jones RB, Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA, Savage CO, Segelmark M, Tesar V, van Paassen P, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med. 2010;363(3):211–20.
Smith RM, Jones RB, Specks U, Bond S, Nodale M, Aljayyousi R, Andrews J, Bruchfeld A, Camilleri B, Carette S, et al. Rituximab as therapy to induce remission after relapse in ANCA-associated vasculitis. Ann Rheum Dis. 2020;79(9):1243–9.
Walsh M, Merkel PA, Peh CA, Szpirt W, Puechal X, Fujimoto S, Hawley CM, Khalidi NA, Flossmann O, Wald A, et al. Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N Engl J Med. 2020;382(7):622–31.
Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Turkiewicz A, Tchao NK, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363(3):221–32.
Park JW, Curtis JR, Moon J, Song YW, Kim S, Lee EB. Prophylactic effect of trimethoprim-sulfamethoxazole for pneumocystis pneumonia in patients with rheumatic diseases exposed to prolonged high-dose glucocorticoids. Ann Rheum Dis. 2018;77(5):644–9.
Raso S, Napolitano M, Arrigo G, Reale F, Lucchesi A, Silimbani P, Maggio A, Calvaruso G, Consoli U, Mannina D, et al. Antimicrobial prophylaxis in patients with immune thrombocytopenia treated with rituximab: a retrospective multicenter analysis. Ann Hematol. 2021;100(3):653–9.
Macy E, Poon KYT. Self-reported antibiotic allergy incidence and prevalence: age and sex effects. Am J Med. 2009;122(8):778.e771-777.
Shehab N, Patel PR, Srinivasan A, Budnitz DS. Emergency department visits for antibiotic-associated adverse events. Clin Infect Dis. 2008;47(6):735–43.
Butalia S, Lewin AM, Simpson SH, Dasgupta K, Khan N, Pilote L, Johnson JA, Ghali WA, Rabi DM. Sex-based disparities in cardioprotective medication use in adults with diabetes. Diabetol Metab Syndr. 2014;6(1):117.
Benson RA, Okoth K, Keerthy D, Gokhale K, Adderley NJ, Nirantharakumar K, Lasserson DS. Analysis of the relationship between sex and prescriptions for guideline-recommended therapy in peripheral arterial disease, in relation to 1-year all-cause mortality: a primary care cohort study. BMJ Open. 2022;12(3):e055952.
Merkel PA, Niles JL, Mertz LE, Lehane PB, Pordeli P, Erblang F. Long-term safety of rituximab in granulomatosis with polyangiitis and in microscopic polyangiitis. Arthritis Care Res (Hoboken). 2021;73(9):1372–8.
Acknowledgements
Not applicable.
Funding
This work was supported by the Fonds de Recherche Santé du Québec [#324105 to AM].
Author information
Authors and Affiliations
Contributions
AM conceived on the study, AM, HB, CSM, SB, and JRC contributed to study design, AM and HB performed the analyses, AM, SB and EV interpreted the results, AM wrote the manuscript with input from all authors, AM, EV, SB, CSM, JCR revised manuscript critically for important intellectual content. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the McGill University Faculty of Medicine Institutional Review Board (22-01-037). The requirement for individual participant consent was waived.
Consent for publication
Not applicable.
Competing interests
JRC reports grants or contracts from Abbvie, Amgen, Aqtual, Bendcare, BMS, CorEvitas, FASTER, GSK, IlluminationHealth, Janssen, Labcorp, Lilly, Myriad, Novartis, Pfizer, Scipher, Setpoint, Tnacity Blue Ocean, UCB, outside of the submitted work, and consulting fees from Abbvie, Amgen, Aqtual, Bendcare, BMS, CorEvitas, FASTER, GSK, IlluminationHealth, Janssen, Labcorp, Lilly, Myriad, Novartis, Pfizer, Scipher, Setpoint, Tnacity Blue Ocean, UCB, outside the submitted work. All other authors have nothing to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table S1.
ICD-9 and ICD-10 inpatient primary diagnosis codes to define serious infection. Supplementary Table S2. Multivariable logistic regression analysis of factors associated with TMP-SMX use within 6 months of index rituximab. Supplementary Table S3. Sex-stratified cohort characteristics overall and according to TMP-SMX use. Supplementary Table S4. Multivariable logistic regression analysis of factors associated with TMP-SMX use, stratified by sex. Supplementary Table S5. Univariable Cox proportional hazards regression for factors associated with time to TMP-SMX discontinuation (N = 389a).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Mendel, A., Behlouli, H., de Moura, C.S. et al. Trimethoprim-sulfamethoxazole prophylaxis during treatment of granulomatosis with polyangiitis with rituximab in the United States of America: a retrospective cohort study. Arthritis Res Ther 25, 133 (2023). https://doi.org/10.1186/s13075-023-03114-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13075-023-03114-7