Abstract
Background
Vitis heyneana is widely distributed in the north of Vietnam, it has been used in Vietnamese traditional medicine as an agent for treatment of arthritis, bronchitis, carbuncles and inflammatory conditions, and menstrual irregularities. However, this plant has not been investigated in phytochemical constituents and biological effects, especially in the anti-inflammatory property.
Results
Bioassay-guided fractionation of the EtOAc soluble fraction from the aerial part of Vitis heyneana resulted in the isolation of a series of oligostilbenoids as piceid (1), 2-r-viniferin (2), betulifol A (3), vitisinol C (4), (-)-trans-ε-viniferin (5), α-viniferin (6), shoreaketon (7), amurensin B (8), vitisinol B (9), and cis-vitisin B (10). Compound 5 showed the most potent inhibitory activities by suppressing LPS-induced COX-2 expression and PGE2 production. This compound exhibited significantly reduced LPS-induced nitric oxide (NO) release in a dose-dependent manner. These effects are accompanied with the inhibition of transcription factor NF-κB activation.
Conclusion
The results suggested that trans-ε-viniferin exerts anti-inflammatory effects via suppression the NF-κB activation in RAW 264.7 cells.

Similar content being viewed by others
Background
The genus Vitis (family Vitaceae) is in the major group with more than 66 species identified and is distributed throughout the world [1]. In Vietnam, 6 species of Vitis genus have been reported until now, including V. larbusca L., V. heyneana Roem, & Schult., V. retordii Roman du Caill. Ex Planch. V. vinifera L., V. balansana Planch., V. flexuosa Thunb [2]. Typical constituents in Vitis genus have been reported to be oligomers of resveratrol named stilbenoids [3,4,5,6,7]. To date, over 1000 stilbenoids have been identified in various plant families such as Celastraceae, Cyperaceae, Fabaceae, Iritaceae, Moraceae, Paeoniaceae, and Vitaceae. The Vitaceae family includes about 900 species within 14–17 genera, primarily in tropical climate [8, 9]. Of these genera, only five, the Ampelopsis, Cissus, Cyphostemma, Parthenocissus, and Vitis genera reported the presence of stilbenoids. However, chemical constituents of the species in Vitis genus have been studied the most. Stilbenoids possessed various pharmacological activities such as antioxidative, anti-inflammatory, and antimicrobial activities, as well as having cardioprotective, hepatoprotective, and neuroprotective effects [10,11,12,13,14]. Although approximately 100 stilbenoid monomers, dimers, and oligomers have been found in all Vitis species, research on the chemical compositions and biological activities of Vitis genus remains lacking [15]. Our screening anti-inflammatory effect of an ethanol extract of 4 Vitis species including V. heyneana Roem. & Schult. (VH), Vitis vinifera L. (VV); Vitis balansana Planch. (VB), and Vitis labrusca L. (VL) collected in Vietnam via suppression of LPS-induced COX-2 expression found that 96% of ethanol extract of VH exhibited the most activity [16]. Besides, V. heyneana is widely distributed in northern Vietnam, for example in Cao Bang, Lang Son, and Lao Cai provinces. The stems and roots of this species are traditionally used for the treatment of arthritis, bronchitis, carbuncles and inflammatory conditions, and menstrual irregularities in Vietnamese indigenous peoples [17]. So far, few studies have been done to investigate the chemical constituents of VH. Currently, only a few studies have referred to the presence of stilbenoids in VH [17,18,19]. However, evaluation of anti-inflammatory activities of the VH species have not been studied yet. This study reports the anti-inflammatory effects of VH extracts and its isolated oligostilbenoids via suppressing LPS-induced COX-2 expression, PGE2 and NO productions, and NF-κB activation in RAW 264.7 macrophages.
Results and discussion
Screening inhibitory activities
To study the cytotoxic effects of extract and its fractions on cell viability, the RAW 264.7 cells were incubated and treated a concentration of all materials (50 µg/mL). The results showed that all the extract and fractions that induced cell toxicities were negligible at the above concentrations [16]. In order to examine candidate extract/fractions inhibiting COX-2 in RAW 264.7 cells, ethanol V. heyneana extract (VH) and its fractions (n-hexane-VHH, ethyl acetate-VHE and water-VHW) were tested on COX-2 mRNA expression level by qPCR. As shown in Fig. 1, the inhibitory effect of COX-2 mRNA was mostly potently suppressed by VHE and VH.
COX-2 expression effects of V. heyneana extract (VH) and its fractions (VHH: n-hexane, VHE: ethyl acetate, VHW: water fraction; all 50 µg/mL). Eighteen hours after treating cells with LPS (5 µg/mL) with or without fractions in RAW 264.7 cells. Samples were harvested and lysated for COX-2 mRNA level by qPCR. Relative changes in the COX-2 mRNA expression (*significant as compared to control, *, p < 0.05; **, p < 0.01; ***, p < 0.001; #significant as compared to LPS group, n = 4–5)
Structure identification of the isolated compounds
To investigate the active components from the potential fraction, several chromatographic techniques were applied and ten compounds were obtained after purification. On the basis of NMR spectroscopic analysis, and in comparison with the previous studies, the chemical structures of these compounds were identified as piceid (1), 2-r-viniferin (2), betulifol A (3), vitisinol C (4), (-)-trans-ε-viniferin (5), α-viniferin (6), shoreaketon (7), amurensin B (8), vitisinol B (9), and cis-vitisin B (10) (Fig. 2) [17, 20,21,22,23,24,25,26].
Screening inhibitory activities of oligostilbenoids by suppressing LPS-induced COX-2 expression and PGE2 production in RAW 264.7 macrophages
The effects of all isolated compounds at concentration of 10 µM, and a reference agent, meloxicam (20 µM) were further examined on LPS-induced COX-2 protein, mRNA expression and LPS-induced PGE2 production in RAW 246.7 cells by reported method with slight modification. As the results in Fig. 3, western blotting and real time-PCR assays revealed that, among the active metabolites, trans-ε-viniferin (5; 10 µM) potently suppressed LPS-induced COX-2 expression in RAW 246.7 cells (Fig. 3a, b). Furthermore, ELISA assay confirmed that 5 most inhibited the level of LPS-induced PGE2 production in RAW 264.7 cells (Fig. 3c).
Comparison of COX-2 protein expression effects of compounds from V. heyneana. 18 h after treating cells with LPS (5 μg/mL) with or without compounds in RAW 264.7 cells, samples were harvested and lysated to immunoblottings with COX-2 and β-actin antibodies. b COX-2 mRNA level by qCPR (***significant as compared to control, *p < 0.05; #significant as compared to LPS group, n = 5. c Comparison of PGE2 production effects of all compounds (10 M). RAW 264.7 cells were incubated with 5 μg/mL LPS for 18 h with or without compounds and amount of PGE2 in medium was determined using PGE2-specific ELISA assays (***significant as compared to control, *p < 0.05; #significant as compared to LPS group, n = 5. Codes: VH02 compound 1; VH03 - 2; VH04 - 3; VH06 - 4; VH07 - 5; VH08 - 6; VH11 - 7; VH13 - 8; VH15 - 9; and VH16 - 10.
Effects of 5 on COX-2, iNOS expression and PGE2 production in RAW 264.7 macrophages
Considering that the 5 was the most active metabolite, the effects of this compound was investigated on LPS-induced PGE2 production in RAW 264.7 cells using ELISA method with slight modification. As the results in Fig. 4a show, stimulation of cells resulted in the increase of PGE2 production compared with unstimulated vehicle cells. In the administration of 5 (1–30 µM), the PGE2 productions were remarkably reduced in a concentration dependent manner (p < 0.05). We further investigated whether the inhibition effect by the same range of concentration of 5 was related to the gene expression, especially in quantitation protein and mRNA level of COX-2 by performing western blotting and real-time PCR. As shown in Fig. 4b and c, COX-2 levels were decreased by both of protein amount and mRNA levels. These results were also confirmed by dose dependent manner.
Effect of 5 on COX-2 and iNOS expression in dose-dependent manner (1–30 µM). Raw264.7 cells were treated with 5 μg/mL LPS for 18 h with or without VH07 and then harvested and lysated to immunoblottings with COX-2, iNOS and β-actin antibodies. b Effect of 5 on LPS-induced COX-2 was analyzed by qPCR (***significant as compared to control, *p < 0.05; #significant as compared to LPS group, n = 5). c Effect of 5 on PGE2 production. RAW 264.7 cells were incubated with 5 μg/mL LPS for 18 h with or without 5 and amounts of PGE2 in medium was determined using PGE2-specific ELISA assays. (***significant as compared to control, *p < 0.05; #significant as compared to LPS group, n = 4)
Effect of 5 on NO productions and NF-κB activation
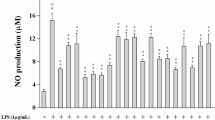
As shown in Fig. 5a, 5 (Code VH07, 1–30 µM) significantly inhibited LPS-induced NO production in a dose dependent manner. Especially, at the highest concentration (30 µM), this compound could decrease the amount of NO production more than 2-fold compared to unstimulated vehicle. Since NF-κB was identified as an important transcription factor that controls several pro-inflammatory mediations, we investigated the NF-κB transcription activity by performing luciferase reporter gene assay and the results are shown in Fig. 5b. Compound 5 (1–30 µg/mL) dose dependent reduced LPS-induced NF-κB transitivity (p < 0.05).
Discussion
Vitis sp. is widely distributed and has been used as the raw material for juice and wine all over the world. For the pharmaceutical application, it has been reported that the roots, stems and leaves possessed anti-inflammatory, antioxidants, and anti-tumour activities, and contains a number of stilbenoid and resveratrol oligomers. Previously, resveratrol (3,4′,5-trihydroxystilbene) isolated mostly from Vitis sp. as a main metabolite with high concentration, was shown to play an important role in human health with extremely extensive bioactivities such as anti-bacterial, anti-thrombotic, anti-oxidation, anti-inflammatory, reduce hypertension, and especially anti-cancer [9,10,11,12,13]. In addition, several studies focused on resveratrol oligomers that are characterized by the polymerization of several resveratrol units [9,10,11,12,13]. In this study, we demonstrated the potential involvement of the NF-κB pathway in the anti-inflammation of metabolites from V. heyneana. Among the active components, 5 was found to be the most anti-inflammatory activity in suppression of NO, PGE2, and COX-2 production in LPS-stimulated RAW 264.7 macrophage cells. We also found that, this oligostilbenoid inhibited LPS-induced NF-κB activation.
RAW 264.7 cells are the murine macrophage cells line that plays an important testable model for anti-inflammatory agent nowadays. When the macrophage cells were activated by LPS, a number of cytokines were released and recordable. Among them, NO is an inflammation mediator, in which, NO produced by iNOS coursed toxicity to cells and directly concern to the pathogenesis of inflammation process. COX-1 and COX-2 are the isozymes that convert arachidonic acid to prostaglandin, however, COX-2 responses mainly to produce a huge amount of PGEs in macrophage cells. Inhibition of the above productions may be the effective method for the treatment of several types of inflammation. Of our experiments, this is the first report confirming that oligostibene from V. heyneana suppresses the LPS-induced inflammatory response by activating NF-κB in vitro. (-)-Trans-ε-viniferin was first isolated from V. vinifera and classified as a model for its bio-synthesis from resveratrol. Similar to resveratrol, this dimer was shown to have several biological properties such as anti-oxidant, antidepressant, and anti-adipogenesis [27,28,29,30]. This compound showed cytotoxicity in several cancer cell lines as C6, Hep G2, HeLa, and MCF-7n and exerted the anti-proliferative and pro-apoptotic effect in U266, RPMI8226, Jurkat, K562 and U937 and other cancer cell lines [4, 31]. (-)-Trans-ε-viniferin and its derivative compounds slightly reduced cell proliferation on human adenocarcinoma colon cells and could constitute new putative anti-cancer agents on colon carcinoma [32]. (-)-Trans-ε-viniferin significantly attenuated mutant Htt-induced depletion of SIRT3 and protected cells from mutant Htt. This compound also decreased levels of reactive oxygen species and prevented loss of mitochondrial membrane potential in cells expressing mutant Htt [33]. The other form, α-viniferin, also down-regulated the LPS-induced expression of pro-inflammatory genes such as iNOS and COX-2 by suppressing the activity of NF-κB via dephosphorylation of Akt/PI3K. This compound suppressed NO and PGE2 production in the late stage of inflammation through induction of heme oxygenase-1 (HO-1), and the expression of pro-inflammatory genes iNOS and COX-2 in the early stage of inflammation by inhibiting the Akt/PI3K-dependent NF-κB activation in BV2 microglial cells [34]. The V. thunbergii extract that was rich in (-)-trans-ɛ-viniferin significantly inhibited PGE2 production in LPS-induced PHCs cells without exhibiting significant cytotoxicity [35].
Even though (-)-trans-ɛ-viniferin and its isomers have been shown to have several anti-inflammatory effects, the molecular mechanism underlying the anti-inflammatory in LPS-induced RAW 264.7 has not been completely elucidated thus far. The results of our research indicate that among the active components, treating the RAW 264.7 macrophage cells with several concentrations of (-)-trans-ɛ-viniferin (5) could inhibit LPS-induced NO, PGE2, iNOS, COX-2 productions in a dose dependent manner. With the highest concentration at 30 µM, this oligostibene significantly reduced the above productions more than 50% as compared to the LPS-treated cell alone.
Methods
Plant materials
The aerial parts of V. heyneana were collected from Lao Cai province (north of Vietnam) in September 2016 and botanically identified by Assoc. Prof. Dr. Nguyen The Cuong, Institute of Ecology and Biological Resources. A voucher specimen (TL07) has been deposited at the Herbarium of IEBR and Department of Phytochemistry, Hanoi, Vietnam.
General experiment procedures
Melting points were determined on an Electrothermal apparatus. Optical rotations were measured on a JASCO V-550 UV/Vis spectrometer (Tokyo, Japan). The NMR [1H (500 MHz), 13C (125 MHz)] experiments were performed on a Bruker Advance 500 spectrometer (United State). Chemical shift was reported in ppm downfield from TMS, with J in Hz. Mass spectra were obtained with an AGILENT 1200 series LC-MSD Ion Trap (United State). Analytical TLC was performed on Kieselgel 60 F254 (Merck) plates (silica gel, 0.25 mm layer thickness) and RP-18 F254 (Merck) plates (0.25 mm layer thickness). UV spots were visualized using ultraviolet irradiation (at 254–365 nm) and by spraying with 10% H2SO4, followed by heating with a heat gun. Column chromatography was performed on silica gel (70–230 and 230–400 mesh, Merck), YMC RP-18 resin (30–50 μm, Fuji Silysia Chemical Ltd.), and Sephadex™ LH-20 columns (Amersham Biosciences, Uppsala, Sweden). Dulbecco’s modified Eagle’s medium (DMEM), trypsin and fetal bovine serum (FBS) were purchased from Gibco BRL (Grand Island, NY). COX-2 (1:1000, Cat: 610204) was obtained from BD Biosciences. Secondary mouse or rabbit HRP-conjugated antibodies (1:5000 or 1:10,000, Cell Signaling, #7074, #7076). β-actin (Cat: A5316), LPS (Cat: L4391), meloxicam (cat: M3935) and MTT (cat: M2128) were purchased from Sigma-Aldrich.
Cell culture
RAW 264.7 cells were purchased from the American Type Culture Collection (ATCC, Rockville, MD). Cells were cultured and growth in DMEM (Gibco) medium containing 10% fetal Bovine Serum (FBS) (Gibco), 100 units/mL penicillin, and 100 μg/mL streptomycin at 37 °C in 5% CO2-95% air. Penicillin and streptomycin were obtained from Biochrom.
Western blot analysis
RAW 264.7 cells were seeded in 6 well-plate and treated with extractions or compounds from V. heyneana. Cells were collected and washed with cold phosphate-buffered saline (PBS). The collected cells were lysed on ice for 30 min in 100 μL lysis buffer [120 mM NaCl, 40 mM Tris (pH 8), 0.1% NP40 (Nonidet P-40)] and centrifuged at 12,000 rpm for 30 min. BCA protein assay kit (Pierce, Rockford, IL) was used to measure protein concentrations as described in a previous report [36]. Finally, bands were detected by ECL kit (Pierce West Femto), and images were acquired using the Odyssey Fc Imager (LI-COR Biosciences).
Quantitative real-time PCR (qRT-PCR)
Total RNA was isolated using Trizol (Takara, Japan) according to the manufacturer’s instructions. COX-2 mRNA expression was analyzed by real-time qPCR (StepOnePlus qPCR cycler, Applied Biosystems) using QuantiFast SYBR Green RT-PCR Kit Qiagen, (#204156) and primers (Qiagen): COX-2 (Ptgs2) (Cat: QT00165347), Mm_GADPH (Cat: QT01658692).
Measurement of nitrite
RAW 264.7 cells (5 × 105 cells) were pre-incubated at 37 °C for 12 h in serum-free medium and NO production was monitored by measuring nitrite levels in culture media using Griess reagent (1% sulfanilamide, 0.1% N-1-naphthylenediamine dihydrochloride, and 2.5% phosphoric acid). Absorbance was measured at 540 nm after incubating for 10 min.
Reporter gene assay
One µg of the plasmid NF-κB or 50 ng of pRL Renilla was transfected into the cells using LipofectAMINE2000 (Invitrogen Corp., Carlsbad, CA) using the Dual-Luciferase Reporter Assay Systems (Promega, Madison, WI, USA). After 6 h, the transfection medium was replaced with the DMEM without serum and the cells were further incubated for 18 h. The firefly and hRenilla luciferase activity was detected using a multilaber counter. Ratio of activity was determined by normalizing the promoter-driven luciferase activity versus hRenilla luciferase.
Enzyme-linked immunosorbent assay (ELISA)
Prostaglandin E2 (PGE2) concentrations in DMEM media were measured by ELISA kit (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s protocols.
Extraction and isolation
The aerial part of V. heyneana (5.0 kg) was extracted with EtOH 96% (3 h × 3L) at 50 °C. The combined extracts were filtered and evaporated under pressure to give green residue (235.5 g) which was suspended in water and partitioned with organic solvent to get n-hexane (26.05 g), EtOAc (129.67 g), and aqueous extract (50.4 g), successfully. The EtOAc extract (34.0 g) was initially chromatographed on a silica gel column (63–200 µM particle size, Merck) eluting with a stepwise gradient of methylene chloride (MC)-MeOH (from 100:1 to 1:100) to yield seven fractions (VHE1-VHE7). Fraction VHE4 (1.5 g) was fractionated by normal-phase silica gel CC (40–63 µM particle size, Merck) eluted with MC-MeOH (20:1) to obtain compound 3 (13 mg) and five smaller fractions (VHE4.1-VHE4.5). Fraction VHE4.3 Fraction VHE4.4 (500 mg) was subjected to Sephadex eluted with a mixture of MeOH-H2O (2:1) to give 4 (23 mg) and 5 (40 mg). Fraction VHE5 (3.5 g) was isolated by silica gel CC with elution mixture of MC-MeOH (15:1) to yield ten sub-fractions (VHE5.1-VHE5.10). Compound 6 (25 mg) was purified from fraction VHE5.3 (400 mg) by using Sephadex LH-20 eluting with MeOH-H2O (5:1). Fraction VHE5.4 (1.1 g) was applied to RP-C18 gel CC using a gradient mixture of MeOH-H2O (from 1:2 to 2:1) to obtain 7 (25 mg), 8 (22 mg), and 9 (13 mg) and eight fractions (VHE5.4.1-VHE5.4.8). Compound 10 (14 mg) was purified from fraction VHE5.4.5 (300 mg) by using MCI gel CC eluting with MeOH-H2O (3:2). Fraction VHE6 (1.3 g) was separated on Sephadex gel CC eluting with MeOH-H2O (3:2) to yield 1 (12 mg), and 2 (25 mg). Structural identification of the isolated compounds 1–10 have been previously published by our group [37, 38].
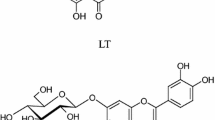
(-)-Trans-ε-viniferin (5): Grey-brown solid; mp150–152 °C, soluble in ethanol, methanol, and acetone; \(\left[ \alpha \right]_{\text{D}}^{25}\) -47.0 (c = 0.5, MeOH); R f = 0,64 (TLC, silica gel, dichlomethane-methanol 7:1, v/v). 1H-NMR (500 MHz, acetone-d6), δH (ppm): 7.20 (2H, d, J = 8.5 Hz, H-2, 6), 7.17 (2H, d, J = 8.5 Hz, H-2′,6′), 6.90 (1H, d, J = 16.5, H-7′), 6.83 (2H, d, J = 8.5 Hz, H-3, 5), 6.73 (2H, d, J = 9.0 Hz, H-3′,5′), 6.72 (1H, brs, H-14ʹ), 6.71 (1H, d, J = 16.5 Hz, H-8′), 6.33 (1H, d, J = 2,0 Hz, H-12′), 6.24 (3H, s, H-10, 12, 14), 5.42 (1H, d, J = 5.5 Hz, H-7), 4.47 (1H, d, J = 5.5 Hz, H-8); 13C-NMR (125 MHz, acetone-d6), δC (ppm): 162.5 (C-11′), 159.9 (C-11, 13), 159.6 (C-13′), 158.2 (C-4, 4′), 147.4 (C-9), 136.4 (C-9′), 133.9 (C-1), 130.1 (C-7′), 129.9 (C-1′), 128.7 (C-2′, 6′), 127.9 (C-2, 6), 123.5 (C-8′), 119.8 (C-10′), 116.3 (C-3′,5′), 116.2 (C-3, 5), 107.0 (C-10, 14)), 104.2 (C-14′), 102.1 (C-12), 96.8 (C-12′), 93.9 (C-7), 57.1 (C-8). ESI–MS m/z: 477 [M + Na]+.
Statistics
Values are presented as mean ± SE unless otherwise stated. P-values were calculated by Student’s t test or one-way analysis of variance followed by Bonferroni post hoc testing using GraphPad Prism 5 (GraphPad Software Inc.). P < 0.05 was considered statistically significant. Data are expressed as mean ± SEM. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Conclusion
This the first time that a Vietnamese V. heyneana extract and its phytochemical constituents have been reported to possess an anti-inflammatory activity. The results demonstrated that one of the most active compounds, (-)-trans-ɛ-viniferin, decreased NO, PGE2, iNOS, and COX-2 productions in RAW 264.7 macrophage cells after LPS stimulation. This anti-inflammatory activity may mediate by the NF-κB activation mechanism in the RAW 264.7 cells. However, to imply that this Vitis sp. and its components may be useful in the prevention or treatment of inflammatory diseases may be premature as further studies and confirmation of these results are required.
References
Royal Botanic Gardens Kew (2013) The plant list: Vitis. http://www.theplantlist.org/browse/A/Vitaceae/Vitis/. Accessed May 2017
Cuong NT (2012) Study on classification of Vitaceae Juss. in Vietnam, Dissertation of Biology, Institute of Ecology & Biological Recourse, Vietnam Academy of Science & Technology
Ha DT, Kim H, Thuong PT, Ngoc TM, Lee I, Hung ND, Bae K (2009) Antioxidant and lipoxygenase inhibitory activity of oligostilbenes from the leaf and stem of Vitis amurensis. J Ethnopharmacol 125(2):304–309
Ha DT, Chen QC, Hung TM, Youn UJ, Ngoc TM, Thuong PT, Kim HJ, Seong YH, Min BS, Bae K (2009) Stilbenes and oligostilbenes from leaf and stem of Vitis amurensis and their cytotoxic activity. Arch Pharm Res 32(2):177–183
Chiou WF, Shen CC, Chen CC, Lin CH, Huang YL (2009) Oligostilbenes from the roots of Vitis thunbergii. Planta Med 75(8):856–859
Nassiri-Asl M, Hosseinzadeh H (2016) Review of the pharmacological effects of Vitis vinifera (Grape) and its bioactive constituents: an update. Phytother Res 30(9):1392–1403
Pawlus AD, Sahli R, Bisson J, Rivière C, Delaunay JC, Richard T, Gomès E, Bordenave L, Waffo-Téguo P, Mérillon JM (2013) Stilbenoid profiles of canes from Vitis and Muscadinia species. J Agric Food Chem 61(3):501–511
Keller M (2010) The science of grapevines: anatomy and physiology. Academic Press, London, p 400p
Soejima A, Wen J (2006) Phylogenetic analysis of the grape family (Vitaceae) based on three chloroplast markers. Am J Bot 93:278–287
Roupe KA, Remsberg CM, Yáñez JA, Davies NM (2006) Pharmacometrics of stilbenes: seguing towards the clinic. Curr Clin Pharmacol 1(1):81–101
Bertelli AA, Das DK (2009) Grapes, wines, resveratrol, and heart health. J Cardiovasc Pharmacol 54(6):468–476
Vidavalur R, Otani H, Singal PK, Maulik N (2006) Significance of wine and resveratrol in cardiovascular disease: french paradox revisited. Exp Clin Cardiol 11:217–225
Pezzuto JM (2008) Grapes and human health: a perspective. J Agric Food Chem 56:6777–6784
Dinicola S, Cucina A, Antonacci D, Bizzarri M (2014) Anticancer effects of grape seed extract on human cancers: a review. J Carcinog Mutagen S 8:005
Alison DP, Pierre WWT, Jonah S, Merillon JM (2012) Stilbenoid chemistry from wine and the genus Vitis, a review. J Int Sci Vigne Vin 46(2):57–111
Long PT, An NTT, Ha DT, Tuan DT, Dung LV (2017) Some stilbenoids isolated from the ethyl acetate fractions of Vitis heyneana Roem. & Schult. Pharmaceutical J 57(493):59–64
Chi VV (2012) Dictionary of Vietnam medicinal plants, vol 2. Medical Publishing House, Hanoi, p 356-357
Li WW, Ding LS, Li BG, Chen YZ (1996) Oligostilbenoids from Vitis heyneana. Phytochemistry 42(4):1163–1165
Li Y, Li ZH, Zhang CH, Zhang XD, Cui ZH (2013) Chemical constituents from Vitis heyneana Roem. & Schult. Biochem Syst Ecol 50:266–268
Teguo PW, Decendit A, Vercauteren J, Vercauteren J, Deffieux G, Mérillon JM (1996) Trans-resveratrol-3-O-β-glucoside (piceid) in cell suspension cultures of Vitis vinifera. Phytochemistry 42(6):1591–1593
Korhammer S, Reniero F, Mattivi F (1995) An oligostilbene from Vitis roots. Phytochemistry 38:1501–1504
Li W, Li B, Chen Y (1998) Oligostilbenes from Vitis betulifolia. Phytochemistry 49:1393–1394
Huang YL, Tsai WJ, Shen CC, Chen CC (2005) Resveratrol derivatives from the roots of Vitis thunbergii. J Nat Prod 68(2):217–220
Kitanaka S, Ikezawa T, Yasukawa K, Yamanouchi S, Takido M, Sung HK, Kim IH (1990) (+)-α-viniferin, an anti-inflammatory compound from Caragana chamlagu root. Chem Pharm Bull 38(2):432–435
Ito T, Furusawa M, Iliya I, Tanaka T, Nakaya KI, Sawa R, Kubota Y, Takahashi Y, Riswan S, Iinuma M (2005) Rotational isomerism of a resveratrol tetramer, shoreaketone, Shorea uliginosa. Tetrahedron Lett 46(17):3111–3114
Huang KS, Lin M (1999) Oligostilbenes from the roots of Vitis amurensis. J Asian Nat Prod Res 2(1):21–28
Privat C, Telo JP, Bernardes-Genisson V, Vieira A, Souchard JP, Nepveu F (2002) Antioxidant properties of trans-epsilon-viniferin as compared to stilbene derivatives in aqueous and nonaqueous media. J Agric Food Chem 50(5):1213–1217
Yáñez M, Fraiz N, Cano E, Orallo F (2006) (-)-Trans-epsilon-viniferin, a polyphenol present in wines, is an inhibitor of noradrenaline and 5-hydroxytryptamine uptake and of monoamine oxidase activity. Eur J Pharmacol 542(1–3):54–60
Ohara K, Kusano K, Kitao S, Yanai T, Takata R, Kanauchi O (2015) ε-viniferin, a resveratrol dimer, prevents diet-induced obesity in mice. Biochem Biophys Res Commun 468(4):877–882
Billard C, Izard JC, Roman V, Kern C, Mathiot C, Mentz F, Kolb JP (2002) Comparative antiproliferative and apoptotic effects of resveratrol, epsilon-viniferin and vine-shots derived polyphenols (vineatrols) on chronic B lymphocytic leukemia cells and normal human lymphocytes. Leuk Lymphoma 43(10):1991–2002
Giovannelli L, Innocenti M, Santamaria AR, Bigagli E, Pasqua G, Mulinacci N (2014) Antitumoural activity of viniferin-enriched extracts from Vitis vinifera L. cell cultures. Nat Prod Res 28(22):2006–2016
Marel AK, Lizard G, Izard JC, Latruffe N, Delmas D (2008) Inhibitory effects of trans-resveratrol analogs molecules on the proliferation and the cell cycle progression of human colon tumoral cells. Mol Nutr Food Res 52(5):538–548
Fu J, ** J, Cichewicz RH, Hageman SA, Ellis TK, **ang L, Peng Q, Jiang M, Arbez N, Hotaling K, Ross CA, Duan W (2012) Trans-(-)-ε-viniferin increases mitochondrial sirtuin 3 (SIRT3), activates AMP-activated protein kinase (AMPK), and protects cells in models of Huntington Disease. J Biol Chem 287(29):24460–24472
Dilshara MG, Lee KT, Kim HJ, Lee HJ, Choi YH, Lee CM, Kim LK, Kim GY (2014) Anti-inflammatory mechanism of α-viniferin regulates lipopolysaccharide-induced release of proinflammatory mediators in BV2 microglial cells. Cell Immunol 290(1):21–29
Tsai CF, Wang KT, Chen LG, Lee CJ, Tseng SH, Wang CC (2014) Anti-inflammatory effects of Vitis thunbergii var. taiwaniana on knee damage associated with arthritis. J Med Food 17(4):479–486
Ha DT, Oh J, Khoi NM, Dao TT, le Dung V, Do TN, Lee SM, Jang TS, Jeong GS, Na M (2013) In vitro and in vivo hepatoprotective effect of ganodermanontriol against t-BHP-induced oxidative stress. J Ethnopharmacol 150(3):875–885
Long PT, An NTT, Ha DT, Tuan DT, Oanh HV, Dung LV (2017) Some stilbenoids isolated from the ethyl acetate fractions of Vitis heyneana Roem & Schult. Pharmaceutical J 493(57):59–63
Long PT, An NTT, Thuy PT, Oanh HV, Khoi NM, Ha DT Oligostilbenoids isolated from the ethyl acetate fractions of Vitis heyneana Roem & Schult. J Med Mat 22(6): 352–352–360
Authors’ contributions
DTH, TTH conceived and designed the study. PTL, NTTA, LTL, HVO completed the extraction, isolation and identified the chemical structures of all isolated compounds. DTT, TMH and NMK carried out the biological experiments, data analysis. TMH, TTH and DTH drafted the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was assisted by a grant from Vietnam National Foundation for Science and Technology Development (NAFOSTED: 106.YS.05-2014.26).
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Not applicable.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
Vietnam National Foundation for Science and Technology Development.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ha, D.T., Long, P.T., Hien, T.T. et al. Anti-inflammatory effect of oligostilbenoids from Vitis heyneana in LPS-stimulated RAW 264.7 macrophages via suppressing the NF-κB activation. Chemistry Central Journal 12, 14 (2018). https://doi.org/10.1186/s13065-018-0386-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-018-0386-5