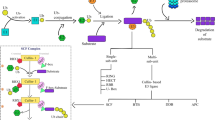
Abstract
Background
Ubiquitination is essential for many cellular processes in eukaryotes, including 26S proteasome-dependent protein degradation, cell cycle progression, transcriptional regulation, and signal transduction. Although numerous ubiquitinated proteins have been empirically identified, their cognate ubiquitin E3 ligases remain largely unknown.
Results
Here, we generate a complete ubiquitin E3 ligase-encoding open reading frames (UbE3-ORFeome) library containing 98.94% of the 1515 E3 ligase genes in the rice (Oryza sativa L.) genome. In the test screens with four known ubiquitinated proteins, we identify both known and new E3s. The interaction and degradation between several E3s and their substrates are confirmed in vitro and in vivo. In addition, we identify the F-box E3 ligase OsFBK16 as a hub-interacting protein of the phenylalanine ammonia lyase family OsPAL1–OsPAL7. We demonstrate that OsFBK16 promotes the degradation of OsPAL1, OsPAL5, and OsPAL6. Remarkably, we find that overexpression of OsPAL1 or OsPAL6 as well as loss-of-function of OsFBK16 in rice displayed enhanced blast resistance, indicating that OsFBK16 degrades OsPALs to negatively regulate rice immunity.
Conclusions
The rice UbE3-ORFeome is the first complete E3 ligase library in plants and represents a powerful proteomic resource for rapid identification of the cognate E3 ligases of ubiquitinated proteins and establishment of functional E3–substrate interactome in plants.
Similar content being viewed by others
Background
Protein interactome analysis enables the comprehensive identification of protein complexes and protein modifications, thus hel** us to understand specific biological mechanisms, elucidate cellular functions, and decipher genotype–phenotype relationships [1, 2]. The yeast two-hybrid (Y2H) system has been widely used to analyze direct protein–protein interactions and to provide high-quality binary interactomes [3]. It has been used to develop high-throughput human binary protein–protein interactome maps, from the first-generation maps comprising 2754 pairwise interactions in 2005 [4, 5] to the third-generation maps comprising 53,000 pairwise interactions in 2020 [1].
Protein ubiquitination is required for a plethora of cellular processes in eukaryotes, including proteasome-dependent protein degradation, cell cycle progression, transcriptional regulation, and signal transduction [6] and is sequentially catalyzed by three different types of enzymes: the ubiquitin-activating enzyme E1, the ubiquitin-conjugating enzyme E2, and the ubiquitin ligase E3. E1 catalyzes the ATP-dependent activation of ubiquitin and the formation of a thioester bond with ubiquitin. E2 binds to the activated ubiquitin and transfers it to its substrate via an E3 ligase [6]. E3 ligases, the most heterogeneous of these enzymes, mediate substrate specificity. Their direct physical interactions with substrates determine the modification mode of the substrates. E3 ligases can be divided into single- and multi-subunit types. The single-subunit type includes HECT (homologous to the E6AP carboxyl terminus), RING (really interesting new gene), and U-box, while the multi-subunit type includes SCF (SKP1–CUL1–F-box), CUL3–BTB, CUL4–DDB1–DWD, and APC/C complex (anaphase-promoting complex/cyclosome), in which F-box, BTB, DWD, and APC co-activator subunits determine substrate specificity [7]. The identification of E3 ligases and their substrates is critical for understanding the protein ubiquitination mediated biological processes.
In the past decade, significant progress has been made in identifying ubiquitinated proteins in animals using a monoclonal antibody that specially recognizes the putative ubiquitination sites diglycine-modified lysines (K-Ɛ-GG) [8,9,10]. Using this antibody, over 20,000 distinct endogenous ubiquitination sites were identified in human cells [11], and 1543 putative ubiquitinated proteins were identified in rice (Oryza sativa L.) leaves with and without treatment with inducers of plant defense responses [14]. These approaches have led to the identification of a huge number of putative ubiquitinated proteins in both animals and plants; however, their cognate E3s remain largely unknown.
Plant genomes encode approximately 1500 E3s; this family has expanded by more than two-fold compared to E3s in mammals and other species [6, 15]. This observation suggests that E3 ligases may be involved in regulating many more biological processes in plants than in other species. Although Y2H screening has been widely used to identify E3–substrate pairs in plants, the efficiency of screening E3 ligase genes is low for several reasons. First, two-thirds of the clones from a conventional cDNA library are not amenable to fusion in frame with the N-terminal GAL4 activation domain required to validate interactions [16]. Second, some E3 ligase genes are expressed only in specific tissues or developmental stages or under some stress conditions [17]. Therefore, a complete E3 ubiquitin ORFeome library is essential for analyzing ubiquitination interactome in plants.
Rice is an important food crop and a model monocot plant [18]. In the current study, a ubiquitin E3 ligase gene (UbE3) library covering 98.94% of the 1515 E3 ligase genes in rice was generated. In addition to the known E3s of four substrates, several new E3s were identified by using the UbE3 library. These interactions and substrate degradation were confirmed by in vitro and in vivo assays. Furthermore, when the phenylalanine ammonia lyases OsPAL1–OsPAL9 were used as baits, only the F-box-type E3 ligase OsFBK16 interacted with OsPAL1–OsPAL7. We further verified that OsFBK16 degrades OsPAL1, OsPAL5, and OsPAL6 in vivo and demonstrated that overexpression of OsPAL1 and OsPAL6 in rice as well as loss-of-function of OsFBK16 enhanced rice blast disease resistance. Thus, our UbE3 library provides a powerful proteomic resource for the global identification of E3 ligases and analysis of ubiquitination interactome and biological networks in plants.
Results
Putative ubiquitinated proteins in rice and annotation of the ubiquitin E3 ligases
We previously identified 1543 proteins containing ubiquitinated sites in two studies [Yeast two-hybrid (Y2H) screening Equal amounts of plasmid DNA harboring each E3 ligase gene were mixed well and transformed into yeast strain Y187. The yeast cells were spread on SD-Leu plates, cultured for 3 days, harvested, and mixed. Glycerol was added to the solution to a concentration of 15% for long-term storage at − 80 °C. Before the screening, auto-activation of each pGBKT7-bait construct was determined by co-transforming yeast strain AH109 with the empty vector pGADT7. The lack of growth of a yeast culture on an SD-Leu-Trp-His-Ade plate indicated that no autoactivation occurred and the culture could be used for screening. We mated yeast strain AH109 carrying the pGBKT7-bait construct with strain Y187 containing plasmids with all the E3 ligase genes in the pGADT7 background at 30 °C with gentle shaking at 37 rpm for 20–24 h. The culture was checked under a microscope until a 3-lobed structure or a shape resembling “Mickey Mouse” appeared. Following centrifugation and re-suspension, the culture was spread onto SD-Leu-Trp-His-Ade plates and incubated at 30 °C for 3–10 days. The clones were picked, transferred to new SD-Leu-Trp-His-Ade plates, and incubated at 30 °C for 3 more days to further confirm the positive interaction. For PCR amplification, individual yeast clones were picked, transferred into ddH2O, and quickly lysed using liquid nitrogen. PCR was performed using lysed yeast as templates, the pGADT7 vector primers AD-F: CTATTCGATGATGAAGATACCCCACCAAACC and AD-R: GTGAACTTGCGGGGTTTTTCAGTATCTACGATT; the PCR products were purified and subjected to sequencing. Co-IP assays were carried out by agro-infiltration of 4-week-old N. benthamiana leaves. Agrobacterium cultures carrying plasmids harboring GFP-tagged OsSKIPa, OsNRPD1aC, NRR, rTGA2.1, OsPAL1, and their cognate E3 ligase genes fused with HA tag were mixed and co-infiltrated into N. benthamiana leaves. Samples were collected at 72 h after agroinfiltration, and total protein was extracted as previously described [48]. Immunoprecipitation was performed with anti-GFP antibody (MBL, D153-11) or anti-HA antibody and protein G agarose beads. Immunoblotting was performed using an anti-GFP antibody (MBL, 598-7) or anti-HA antibody (MBL, M18907). Protein degradation experiments were performed via transient protein expression in N. benthamiana leaves [49, 50] and rice protoplasts. Agrobacterium cultures carrying plasmids harboring GFP-tagged OsSKIPa, OsNRPD1aC, NRR, rTGA2.1, OsPAL1, and their cognate E3 ligase genes fused with HA tag were mixed and co-infiltrated into N. benthamiana leaves. After 48 h, 50 μM MG132 (Millipore) or an equal volume of DMSO solution was infiltrated in the leaves, which were collected for protein extraction 24 h after MG132 treatment. For the protein degradation assay in rice protoplasts, plasmids harboring GFP-tagged NRR or rTGA2.1 and their cognate E3 ligase genes fused with HA tag as well as the control GUS-HA construct were co-expressed in Nipponbare protoplasts. After 16 h, 50 μM MG132 or an equal volume of DMSO solution was added, and 4 h later, the protoplasts were collected for protein extraction. Protein abundance was detected by immunoblotting using anti-HA or anti-GFP antibody. ACTIN (Abmart, M20009L) or HSP was used as an internal protein control. OsSKIPa, OsNRPD1aC, or OsPAL1 transcript levels were measured by RT-PCR, and ACTIN was used as the internal control. The full-length CDS of OsRING77, OsRING113, OsRFPH2-10, P3IP1, and OsPUB46 were individually fused to the C-terminus of MBP in the pMal-C2X vector, the full-length OsRING116 was fused to the C-terminus of GST in the pGEX6p-1 vector, and the fusion proteins were expressed in Escherichia coli strain BL21. For RING-type E3 ligases, ubiquitination reaction mixtures containing E1 (wheat E1), E2 (AtUBC8), 2 μg/μL ubiquitin (U-100At, Boston Biochem, USA), and purified MBP-OsRING77, MBP-OsRING113, or MBP-OsRFPH2-10 were mixed in 1× reaction buffer (50 mM Tris-HCl, pH 7.4, 10 mM MgCl2, 5 mM ATP, and 2 mM DTT). The reactions were incubated at 30 °C for 2 h, and in vitro E3 ligase activity was determined using an anti-Ub antibody (Millipore, 05-944) and anti-MBP antibody (Abbkine, A02070-2) [51]. For U-box-type E3 ubiquitin ligase, purified MBP-P3IP1 or MBP-OsPUB46 was individually pre-incubated in total rice extracts prior to the assay [52]. For the substrate ubiquitination assay, equal amounts of purified GST-OsSKIPa or GST-OsNRPD1aC were added to the reaction mixture, and ubiquitination was measured using anti-Ub, anti-MBP, or anti-GST (BPI, AbM59001-2H5-PU) antibodies. The full-length coding fragments of OsPAL1 and OsPAL6 were amplified from the rice cultivar NPB and inserted into the pRHV-cGFP vector driven by the maize ubiquitin promoter to generate the OsPAL1 and OsPAL6 overexpression constructs [53]. The generated constructs were introduced into the calli of NPB via Agrobacterium tumefaciens-mediated transformation as described previously [54]. The osfkb16 mutants were generated via CRISPR-Cas9 technology [55]. OsPAL1 and OsPAL6 overexpression lines were identified by quantitative real-time polymerase chain reaction (qRT-PCR) and the mutations in the osfkb16 mutants were analyzed by sequencing. For punch inoculation with M. oryzae, isolate RB22 was cultivated on an oat medium in darkness for 1 week at room temperature and then moved to light for spore induction. After 7–10 days, spore suspension (5 × 105 spores/mL) of RB22 in 0.025% (v/v) Tween 20 was used for punch inoculation on the second leaf (from the top) of 6-week-old plants as previously described [56]. Disease symptoms on leaves were scored 14 days after inoculation. Relative fungal biomass was calculated by measuring the expression of the M. oryzae MoPot2 with the DNA-based quantitative PCR assay. The following are the accession numbers of the genes used in this study: OsFBX466 (LOC_Os02g38499), OsFBX55 (LOC_Os02g38589), OsFBO24 (LOC_Os12g05609), OsFBX481 (LOC_Os12g05709), OsRING62 (LOC_Os02g35347), OsRING66 (LOC_Os02g35365), OsRING202 (LOC_Os11g04280), OsRING203 (LOC_Os11g04281), OsRING77 (LOC_Os02g19140), OsRING113 (LOC_Os03g26370), OsRING171 (LOC_Os01g58400), OsRING394 (LOC_Os12g04590), OsRING199 (LOC_Os11g04680), OsFBX503 (LOC_Os07g17570), OsRING344 (LOC_Os08g42640), OsRING336 (LOC_Os05g41520), OsRING176 (LOC_Os01g58780), OsRING337 (LOC_Os09g12720), OsRING375 (LOC_Os04g22240), BTBT3 (LOC_Os11g37520), HBTB8 (LOC_Os12g08720), OsPUB28 (LOC_Os01g67500), OsPUB46 (LOC_Os04g34140), OsPUB49 (LOC_Os10g41220), OsPUB69 (LOC_Os08g13780), OsFBX68 (LOC_Os02g56810), OsFBX82 (LOC_Os03g20500), OsFBX389 (LOC_Os10g35920), BTBZ1 (LOC_Os01g66890), MBTB47 (LOC_Os10g29180), OsRING116 (LOC_Os01g38700), and OsFBK16 (LOC_Os06g39370).Co-immunoprecipitation (Co-IP) assay
Protein degradation assay in planta
E3 ligase activity and ubiquitination assay in vitro
Rice transformation and M. oryzae inoculation
Accession numbers of the genes used in this study
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files. The rice UbE3 yeast two-hybrid library is available under NCBI with the BioProject ID: PRJNA841249 [57].
Change history
20 July 2022
Figure 2A and 5H have been updated.
References
Luck K, Kim DK, Lambourne L. A reference map of the human binary protein interactome. Nature. 2020;580:402–8.
McWhite CD, Papoulas O, Drew K, Cox RM, June V, Dong OX, et al. A pan-plant protein complex map reveals deep conservation and novel assemblies. Cell. 2020;181:460–474.e414.
Yu H, Braun P, Yildirim MA, Lemmens I, Venkatesan K, Sahalie J, et al. High-quality binary protein interaction map of the yeast interactome network. Science. 2008;322:104–10.
Stelzl U, Worm U, Lalowski M, Haenig C, Brembeck FH, Goehler H, et al. A human protein-protein interaction network: a resource for annotating the proteome. Cell. 2005;122:957–68.
Rual JF, Venkatesan K, Hao T, Hirozane-Kishikawa T, Dricot A, Li N, et al. Towards a proteome-scale map of the human protein-protein interaction network. Nature. 2005;437:1173–8.
Buetow L, Huang DT. Structural insights into the catalysis and regulation of E3 ubiquitin ligases. Nat Rev Mol Cell Biol. 2016;17:626–42.
Vierstra RD. The ubiquitin-26S proteasome system at the nexus of plant biology. Nat Rev Mol Cell Biol. 2009;10:385–97.
Xu G, Paige JS, Jaffrey SR. Global analysis of lysine ubiquitination by ubiquitin remnant immunoaffinity profiling. Nat Biotechnol. 2010;28:868–73.
Kim W, Bennett EJ, Huttlin EL, Guo A, Li J, Possemato A, et al. Systematic and quantitative assessment of the ubiquitin-modified proteome. Mol Cell. 2011;44:325–40.
Wagner SA, Beli P, Weinert BT, Nielsen ML, Cox J, Mann M, et al. A proteome-wide, quantitative survey of in vivo ubiquitylation sites reveals widespread regulatory roles. Mol Cell Proteomics. 2011;10:M111.013284.
Udeshi ND, Svinkina T, Mertins P, Kuhn E, Mani DR, Qiao JW, et al. Refined preparation and use of anti-diglycine remnant (K-ε-GG) antibody enables routine quantification of 10,000s of ubiquitination sites in single proteomics experiments. Mol Cell Proteomics. 2013;12:825–31.
**e X, Kang H, Liu W, Wang GL. Comprehensive profiling of the rice ubiquitome reveals the significance of lysine ubiquitination in young leaves. J Proteome Res. 2015;14:2017–25.
Chen XL, **e X, Wu L, Liu C, Zeng L, Zhou X, et al. Proteomic analysis of ubiquitinated proteins in rice (Oryza sativa) after treatment with pathogen-associated molecular pattern (PAMP) elicitors. Front Plant Sci. 2018;9:1064.
Akimov V, Barrio-Hernandez I, Hansen SVF, Hallenborg P, Pedersen AK, Bekker-Jensen DB, et al. UbiSite approach for comprehensive map** of lysine and N-terminal ubiquitination sites. Nat Struct Mol Biol. 2018;25:631–40.
Hua Z, Vierstra RD. The cullin-RING ubiquitin-protein ligases. Annu Rev Plant Biol. 2011;62:299–334.
Lee S, Lee I, Jung Y, McConkey D, Czerniak B. In-frame cDNA library combined with protein complementation assay identifies ARL11-binding partners. PLoS One. 2012;7:e52290.
Wierbowski SD, Vo TV, Falter-Braun P, Jobe TO, Kruse LH, Wei X, et al. A massively parallel barcoded sequencing pipeline enables generation of the first ORFeome and interactome map for rice. Proc Natl Acad Sci U S A. 2020;117:11836–42.
Izawa T, Shimamoto K. Becoming a model plant: the importance of rice to plant science. Trends Plant Sci. 1996;1:95–9.
He D, Li M, Damaris RN, Bu C, Xue J, Yang P. Quantitative ubiquitylomics approach for characterizing the dynamic change and extensive modulation of ubiquitylation in rice seed germination. Plant J. 2020;101:1430–47.
Zhu L, Cheng H, Peng G, Wang S, Zhang Z, Ni E, et al. Ubiquitinome profiling reveals the landscape of ubiquitination regulation in rice young panicles. Genom Proteom Bioinf. 2020;18:305–20.
Goff SA, Ricke D, Lan TH, Presting G, Wang R, Dunn M, et al. A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science. 2002;296:92–100.
Marín I. Evolution of plant HECT ubiquitin ligases. PLoS One. 2013;8:e68536.
Eloy NB, de Freitas LM, Ferreira PCG, Inzé D. The role of the anaphase-promoting complex/cyclosome in plant growth. Crit Rev Plant Sci. 2015;34:487–505.
Lim SD, Yim WC, Moon JC, Kim DS, Lee BM, Jang CS. A gene family encoding RING finger proteins in rice: their expansion, expression diversity, and co-expressed genes. Plant Mol Biol. 2010;72:369–80.
Jain M, Nijhawan A, Arora R, Agarwal P, Ray S, Sharma P, et al. F-box proteins in rice. Genome-wide analysis, classification, temporal and spatial gene expression during panicle and seed development, and regulation by light and abiotic stress. Plant Physiol. 2007;143:1467–83.
Hua Z, Zou C, Shiu SH, Vierstra RD. Phylogenetic comparison of F-Box (FBX) gene superfamily within the plant kingdom reveals divergent evolutionary histories indicative of genomic drift. PLoS One. 2011;6:e16219.
Xu G, Ma H, Nei M, Kong H. Evolution of F-box genes in plants: different modes of sequence divergence and their relationships with functional diversification. Proc Natl Acad Sci U S A. 2009;106:835–40.
Zeng LR, Park CH, Venu RC, Gough J, Wang GL. Classification, expression pattern, and E3 ligase activity assay of rice U-box-containing proteins. Mol Plant. 2008;1:800–15.
Gingerich DJ, Hanada K, Shiu SH, Vierstra RD. Large-scale, lineage-specific expansion of a bric-a-brac/tramtrack/broad complex ubiquitin-ligase gene family in rice. Plant Cell. 2007;19:2329–48.
Lee J-H, Terzaghi W, Gusmaroli G, Charron J-BF, Yoon H-J, Chen H, et al. Characterization of Arabidopsis and rice DWD proteins and their roles as substrate receptors for CUL4-RING E3 ubiquitin ligases. Plant Cell. 2008;20:152–67.
Yu Y, Zhang H, Long Y, Shu Y, Zhai J. Plant Public RNA-seq Database: a comprehensive online database for expression analysis of ~45 000 plant public RNA-Seq libraries. Plant Biotechnol J. 2022. https://doi.org/10.1111/pbi.13798.
Ma J, Wang Y, Ma X, Meng L, **g R, Wang F, et al. Disruption of gene SPL35, encoding a novel CUE domain-containing protein, leads to cell death and enhanced disease response in rice. Plant Biotechnol J. 2019;17:1679–93.
Liu L, ** L, Huang X, Geng Y, Li F, Qin Q, et al. OsRFPH2-10, a ring-H2 finger E3 ubiquitin ligase, is involved in rice antiviral defense in the early stages of rice dwarf virus infection. Mol Plant. 2014;7:1057–60.
Hou X, **e K, Yao J, Qi Z, **ong L. A homolog of human ski-interacting protein in rice positively regulates cell viability and stress tolerance. Proc Natl Acad Sci U S A. 2009;106:6410–5.
Zhang C, Wei Y, Xu L, Wu KC, Yang L, Shi CN, et al. A bunyavirus-inducible ubiquitin ligase targets RNA polymerase IV for degradation during viral pathogenesis in rice. Mol Plant. 2020;13:836–50.
Chern M, Canlas PE, Fitzgerald HA, Ronald PC. Rice NRR, a negative regulator of disease resistance, interacts with Arabidopsis NPR1 and rice NH1. Plant J. 2005;43:623–35.
Chern M, Bai W, Ruan D, Oh T, Chen X, Ronald PC. Interaction specificity and coexpression of rice NPR1 homologs 1 and 3 (NH1 and NH3), TGA transcription factors and negative regulator of resistance (NRR) proteins. BMC Genomics. 2014;15:461.
Wang R, Wang GL, Ning Y. PALs: emerging key players in broad-spectrum disease resistance. Trends Plant Sci. 2019;24:785–7.
Zhou X, Liao H, Chern M, Yin J, Chen Y, Wang J, et al. Loss of function of a rice TPR-domain RNA-binding protein confers broad-spectrum disease resistance. Proc Natl Acad Sci U S A. 2018;115:3174–9.
Coyaud E, Mis M, Laurent EM, Dunham WH, Couzens AL, Robitaille M, et al. BioID-based identification of Skp Cullin F-box (SCF)β-TrCP1/2 E3 ligase substrates. Mol Cell Proteomics. 2015;14:1781–95.
Lee KA, Hammerle LP, Andrews PS, Stokes MP, Mustelin T, Silva JC, et al. Ubiquitin ligase substrate identification through quantitative proteomics at both the protein and peptide levels. J Biol Chem. 2011;286:41530–8.
Wang L, Wang B, Jiang L, Liu X, Li X, Lu Z, et al. Strigolactone signaling in Arabidopsis regulates shoot development by targeting D53-Like SMXL repressor proteins for ubiquitination and degradation. Plant Cell. 2015;27:3128–42.
Zhou F, Lin Q, Zhu L, Ren Y, Zhou K, Shabek N, et al. D14-SCF(D3)-dependent degradation of D53 regulates strigolactone signalling. Nature. 2013;504:406–10.
Jiang L, Liu X, **ong G, Liu H, Chen F, Wang L, et al. DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature. 2013;504:401–5.
Zhang X, Gou M, Liu CJ. Arabidopsis Kelch repeat F-box proteins regulate phenylpropanoid biosynthesis via controlling the turnover of phenylalanine ammonia-lyase. Plant Cell. 2013;25:4994–5010.
Huang J, Gu M, Lai Z, Fan B, Shi K, Zhou YH, et al. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010;153:1526–38.
Li N, Lin B, Wang H, Li X, Yang F, Ding X, et al. Natural variation in ZmFBL41 confers banded leaf and sheath blight resistance in maize. Nat Genet. 2019;51:1540–8.
Wang R, Ning Y, Shi X, He F, Zhang C, Fan J, et al. Immunity to rice blast disease by suppression of effector-triggered necrosis. Curr Biol. 2016;26:2399–411.
Wang P, Nolan TM, Clark NM, Jiang H, Montes-Serey C, Guo H, et al. The F-box E3 ubiquitin ligase BAF1 mediates the degradation of the brassinosteroid-activated transcription factor BES1 through selective autophagy in Arabidopsis. Plant Cell. 2021;33:3532–54.
Tian W, Wang R, Bo C, Yu Y, Zhang Y, Shin GI, et al. SDC mediates DNA methylation-controlled clock pace by interacting with ZTL in Arabidopsis. Nucleic Acids Res. 2021;49:3764–80.
Ning Y, Jantasuriyarat C, Zhao Q, Zhang H, Chen S, Liu J, et al. The SINA E3 ligase OsDIS1 negatively regulates drought response in rice. Plant Physiol. 2011;157:242–55.
Wang J, Qu B, Dou S, Li L, Yin D, Pang Z, et al. The E3 ligase OsPUB15 interacts with the receptor-like kinase PID2 and regulates plant cell death and innate immunity. BMC Plant Biol. 2015;15:49.
He F, Zhang F, Sun W, Ning Y, Wang GL. A versatile vector toolkit for functional analysis of rice genes. Rice. 2018;11:27.
Fang H, Shen S, Wang D, Zhang F, Zhang C, Wang Z, et al. A monocot-specific hydroxycinnamoylputrescine gene cluster contributes to immunity and cell death in rice. Sci Bull. 2021;66:2381–93.
Lu Y, Ye X, Guo R, Huang J, Wang W, Tang J, et al. Genome-wide targeted mutagenesis in rice using the CRISPR/Cas9 system. Mol Plant. 2017;10:1242–5.
Wang J, Wang R, Fang H, Zhang C, Zhang F, Hao Z, et al. Two VOZ transcription factors link an E3 ligase and an NLR immune receptor to modulate immunity in rice. Mol Plant. 2021;14:253–66.
Wang R, You X, Zhang C, Fang H, **e Q, Wang G-L, Ning Y. An ORFeome of rice E3 ubiquitin ligases for global analysis of the ubiquitination interactome. NCBI BioProject. 2022. https://www.ncbi.nlm.nih.gov/bioproject/PRJNA841249.
Acknowledgements
We thank Dr. Guoqiang Xu at Soochow University for the helpful discussions.
Peer review information
Wen**g She was the primary editor of this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Review history
The review history is available as Additional file 7.
Funding
This project was supported by grants from the National Natural Science Foundation of China (31822041, 32161143009, and 31972225) to Y. N. and the National Natural Science Foundation of China (32001858 and U20A2021) to R.W.
Author information
Contributions
R.W., X. Y., C. Z., H. F., M. W., F. Z., H. K., J. W., Q. Z., X. W., Z. H., F. H., H. T., L. F., X. X., D.W., Z. L., J. W., M. Q., T. Z., P. Z., H. X., and Y. X. performed the experiments. R.W., X. Y., C. Z., H. F., Q. X., W. L. G.-L.W., and Y. N. analyzed the data. R.W. and Y. N. designed the project and wrote the manuscript. All authors participated in the discussion and revision of the manuscript. The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
13059_2022_2717_MOESM1_ESM.ppt
Additional file 1. Figure S1. Analysis of the ubiquitinated site-containing proteins in rice from published studies. Figure S2. Location of all E3 ligase encoding genes on the rice chromosome. Figure S3. Different types of ubiquitin E3 ligase-encoding genes in rice and the number of RT-PCR cloned and chemically synthesized E3 genes in this study. Figure S4. Confirmation of the interaction between OsUBC14 and its candidate E3s. Figure S5. E3 ubiquitin ligase activity of OsRING77, OsRING113, OsPUB28, OsPUB46, OsPUB49 and OsPUB69 in vitro. Figure S6. Ubiquitination assay of GST-OsSKIPa by MBP-OsPUB46. Figure S7. E3 ubiquitin ligase activity of OsRFPH2-10, P3IP1 and OsRING336 in vitro. Figure S8. The E3 ubiquitin ligase activity of OsRING116 in vitro and ubiquitination analysis of rTGA2.1. Figure S9. Transcript level of OPAL1 in individual overexpression lines and the editing types of OsFBK16. Figure S10. Degradation assay of OsPAL5 and OsPAL6 by OsFBK16 in vivo. Figure S11. Transcript level of OsPAL6 in individual overexpression lines.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, R., You, X., Zhang, C. et al. An ORFeome of rice E3 ubiquitin ligases for global analysis of the ubiquitination interactome. Genome Biol 23, 154 (2022). https://doi.org/10.1186/s13059-022-02717-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13059-022-02717-8