Abstract
Breast cancer continues to pose a substantial worldwide health concern, demanding a thorough comprehension of the complex interaction between cancerous cells and the immune system. Recent studies have shown the significant function of exosomes in facilitating intercellular communication and their participation in the advancement of cancer. Tumor-derived exosomes have been identified as significant regulators in the context of breast cancer, playing a crucial role in modulating immune cell activity and contributing to the advancement of the illness. This study aims to investigate the many effects of tumor-derived exosomes on immune cells in the setting of breast cancer. Specifically, we will examine their role in influencing immune cell polarization, facilitating immunological evasion, and modifying the tumor microenvironment. Furthermore, we explore the nascent domain of exosomes produced from immune cells and their prospective involvement in the prevention of breast cancer. This paper focuses on new research that emphasizes the immunomodulatory characteristics of exosomes produced from immune cells. It also explores the possibility of these exosomes as therapeutic agents or biomarkers for the early identification and prevention of breast cancer. The exploration of the reciprocal connections between exosomes formed from tumors and immune cells, together with the rising significance of exosomes derived from immune cells, presents a potential avenue for the advancement of novel approaches in the field of breast cancer therapy and prevention.
Similar content being viewed by others
Introduction
Breast cancer (BC) is the most common cancer among women in most countries, with the highest mortality and morbidity rates [1]. Despite the earlier detection and improved clinical management, there is still a high death rate among women because of treatment failure and metastasis [2]. BC can be divided based on its molecular characteristics into luminal A, luminal B, HER2, estrogen, and progesterone-positive receptors, and triple-negative BC (TNBC). Further subcategories of TNBC include basal-like 1 and 2, immunomodulatory, luminal androgen receptor, mesenchymal, and mesenchymal stem cell-like [3, 4]. TNBC and HER2-positive breast cancers are more likely to metastasize to other areas of the body, such as the brain, bone, lung, liver, and abdominal cavity [5, 6].
In recent decades, there has been a dramatic increase in the literature associated with the involvement of the immune system in BC [7]. It is well known that the immune system plays an important role in the development, progression, and control of BC [8]. The complex relationship between the immune system and cancer cells is characterized by the theory of “cancer immunoediting” (CI) [9], which was initially developed by Dunn et al. [10] CI postulates a dual role of the immune system in cancer: host-protective and tumor-promoting actions [11]. CI consists of “three Es”: elimination, equilibrium, and escape [12, 13]. In the ‘elimination’ phase, all tumor cells may be destroyed by the immune system [13]. During the second phase, the opposing forces remain balanced and cancer growth is still under control. Escape is the terminal stage of immunoediting, in which cancer cell variants effectively evade immune pressure, replicate progressively, and become clinically apparent [13]. In light of this, manipulating the immune system and boosting its defenses against cancer may be one of the keys to controlling cancer [14]. This idea is known as cancer immunotherapy [14]. Exosomes (Exo) have emerged as potential sources of biomarkers for early detection and prognosis in breast cancer. Several studies have identified specific molecules, such as proteins, nucleic acids, and microRNAs, within exosomes that show promise as diagnostic and prognostic markers. MiRNAs are small non-coding RNA molecules that regulate gene expression. For example, miRNA-21, miRNA-155, and miRNA-210 have been found to be upregulated in breast cancer-derived exosomes and are associated with tumor growth, metastasis, and poor prognosis [15,16,46]. However, under certain conditions, the biogenesis of exosomes can occur via an ESCRT-independent pathway. This process can be mediated by lipids such as ceramides and cholesterol [47].
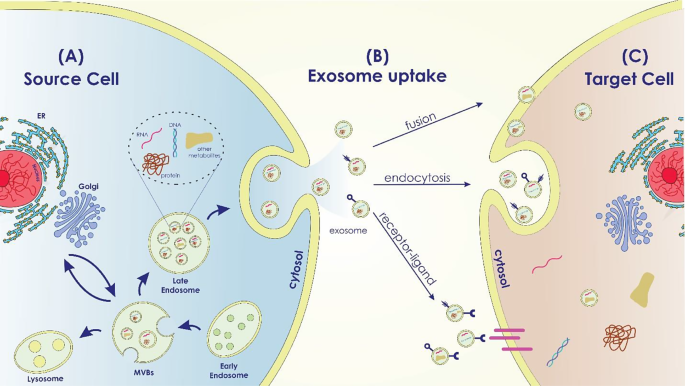
Exosomes participate in several normal and pathological conditions, such as pregnancy, immune responses, cardiovascular disorders, viral pathogenesis, neurological disorders, and cancer [48]. According to the origin of the donor cell, exosomes play a variety of roles, including immune response, antigen presentation, programmed cell death, angiogenesis, inflammation, coagulation, etc. [27, 49] (Figure1).
Exosome Biogenesis in the Source Cell, Uptake, and Cargo Transfer (a) Exosome Biogenesis in the Source Cell: The intracellular mechanisms involved in exosome biogenesis within the source cell are depicted in this area of the image. It displays the early endosome formation process, the sorting of different cargo molecules into the develo** exosomes, and the inward budding of the endosomal membrane to generate multivesicular bodies (MVBs). Labeled cargo molecules consist of proteins, RNAs (miRNAs and mRNAs), lipids, and other bioactive compounds. (b) Exosome Uptake Routes by Target Cells: This portion of the diagram shows the various ways that target cells can absorb exosomes. It demonstrates a variety of exosome uptake processes, including as receptor-mediated interactions, membrane fusion, and endocytosis. (c) Cargo Transfer and Impacts in the Target Cell: The cargo transfer that occurs when exosomes enter the target cell and the ensuing impacts are the main topics of this portion of the picture. It shows how certain cellular processes are activated and how exosome cargo is released into the target cell’s cytoplasm
TDEs effect on immune cells
Over the past 10 years, the study of exosomes has observed increasing attention, representing a high-potential area for their application in cancer as biomarkers and therapeutic tools [50]. Tumor-derived exosomes (TDEs) are important immune adjusters in the tumor milieu. TDEs reduce immune cell cytotoxicity in the microenvironment of tumor cells by transporting suppressive cargo to these cells, to facilitate tumorigenesis [51]. TDEs have been implicated in all stages of BC progression (tumor initiation, growth, dissemination, and metastatic spread) [2]. As part of their immunological functions, exosomes modulate antigen presentation, immune activation, immune surveillance, intercellular communication, and immune suppression [52, 53]. On the other hand, the expression of PD-1 on the surface of BC-derived exosomes can blunt T-cell activation, effectively enabling tumor cells to escape immune surveillance [84].
Precision BC treatment has been significantly improved by nanoparticle-based technology. Exosomes are natural nanoparticles carrying chemotherapeutic drugs, photosensitizers or antitumor drugs. For cancer therapy, exosomes are particularly appealing drug delivery system due to their excellent biosafety, low immunogenicity, carrier properties, and nanoscale penetration effect [Drug delivery carriers Exosomes can be utilized to transfer conventional clinical chemotherapy drugs to targeted sites, thus reducing their toxicity and improving their enrichment effect [126]. Tran et al. loaded aspirin, as an anti-cancer agent, into exosomes that enhanced drug dissolution and homing targeting effect. Aspirin-loaded exosomes exerts cytotoxicity effects on breast and colorectal cancers [127]. Yu et al. showed that erastin-loaded exosomes labeled with folic acid suppressed the proliferation and migration of MDA-MB-231 cells and induced ferroptosis through the depletion of intracellular glutathione and activation of ROS [88]. In another study, exosomes loaded with paclitaxel (PTX) inhibited tumor progression of MDA-MB-231 cells and were considered an effective drug delivery carrier for BC [128]. Doxorubicin (DOX), a common chemotherapeutic remedy for breast cancer, has been shown to decrease the risk of relapse by up to 8% and death by 6.5%. However, the side effects of congestive heart failure and medicine resistance necessitate switching patients taking doxorubicin to fewer effective therapies. Tian et al. engineered immature DCs (imDCs) expressing Lamp2b, an exosomal membrane protein, fused to αv integrin-specific iRGD peptide. Exosome derived from imDCs loaded with DOX and targeted to αv integrin- positive BC cells. iRGD exosomes efficiently inhibited tumor growth without noticeable toxicity [129]. Li et al. introduced milk exosomes containing DOX to the BC cells with specifically CD44-targeting hyaluronic acid (HA). The HA-mExo-Dox effectively targeted CD44-expressed BC cells and induced apoptosis in vitro [130]. Additionally, Hadla and colleagues confirmed that, in comparison to the free drug, DOX-encapsulated exosomes had less of an impact on cardiac toxicity and other tissues. As a result, the dose of DOX can be enhanced, resulting in a targeted cytotoxic effect on breast cancer cells [126].
Clinical implications
Achieving an optimal balance between maximal effectiveness and minimum side effects for conventional drugs used to combat tumors is often difficult due to tumor heterogeneity and biological barriers. Many anti-cancer agents in clinical practice have poor bioavailability and little in vivo stability, causing adverse damage to normal cells. Technologies based on exosomes offer exciting approaches to diagnosing and treating BC. Therapeutic agents can be delivered more efficiently using nanoplatforms, which are innovative dosage forms. Besides uploading drugs, nanoplatforms have the ability to target active molecules as well [131].
Nanoplatforms are mainly used to target tumors by enhancing the permeability and retention (EPR) of tumor internals or by interacting with antigens overexpressed on tumor surfaces. A unique carrier property and biosafety make exosomes ideal for develo** precisely targeted approaches. In this review, current methods of exosomes in BC treatment are discussed specifically, but there are several challenges to overcome. The production and isolation methods need optimization to obtain sufficient quantities of exosomes with consistent quality and purity. Standardization of isolation, characterization, and quality control methods is crucial for safety and reproducibility. Determining the best administration route is challenging, and different routes need evaluation for effectiveness and safety. Achieving targeted delivery to specific tissues or cells is difficult, but strategies like engineering exosomes with specific ligands are being explored. Loading therapeutic cargo into exosomes is challenging due to limited capacity, and efficient methods are needed for stability and preservation. Exosomes from different cell sources may have varying immunogenicity profiles, so minimizing immune responses is important. Lastly, long-term safety and potential off-target effects or accumulation concerns require careful evaluation [132, 133].
However, through genetic editing, researchers can precisely manipulate specific genes to increase or decrease their expression, thus enhancing anti-tumor exosome production. Exosome surface modification during nanocarrier production can improve exosome recruitment and abundance at tumor locations as well as the recognition of a specific target. Additionally, chemotherapeutics and other therapeutic chemicals like phototherapy and photothermal therapy can be delivered using exosomes as a delivery vehicle. On the other hand, the majority of the current studies involve cell and animal research, and are now in the preclinical stage. It is required to do extensive research on the creation of exosome carriers, how they actually work in the human body, and the management of side effects to establish the combination of both the efficacy and safety of exosome delivery as a way to finally achieve clinical use.
Data availability
Not applicable.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68(6):394–424.
Giordano C, La Camera G, Gelsomino L, Barone I, Bonofiglio D, Andò S, Catalano S. The biology of exosomes in breast cancer progression: dissemination, immune evasion and metastatic colonization. Cancers. 2020;12(8):2179.
Gupta P, Neupane YR, Parvez S, Kohli K. Recent advances in targeted nanotherapeutic approaches for breast cancer management. Nanomedicine. 2021;16(29):2605–31.
Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P et al. Breast cancer (primer) Nat. Rev Dis Primers. 2019;66.
Cheang MC, Martin M, Nielsen TO, Prat A, Voduc D, Rodriguez-Lescure A, et al. Defining breast cancer intrinsic subtypes by quantitative receptor expression. Oncologist. 2015;20(5):474–82.
Wong GL, Abu Jalboush S, Lo H-W. Exosomal MicroRNAs and organotropism in breast cancer metastasis. Cancers. 2020;12(7):1827.
Amens JN, Bahçecioglu G, Zorlutuna P. Immune System effects on breast Cancer. Cell Mol Bioeng. 2021;14(4):279–92.
Emens LA. Breast cancer immunobiology driving immunotherapy: vaccines and immune checkpoint blockade. Expert Rev Anticancer Ther. 2012;12(12):1597–611.
Smyth MJ, Dunn GP, Schreiber RD. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and sha** tumor immunogenicity. Adv Immunol. 2006;90:1–50.
Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–8.
Emens LA. Breast Cancer Immunotherapy: facts and HopesBreast Cancer Immunotherapy. Clin Cancer Res. 2018;24(3):511–20.
Edechi CA, Ikeogu N, Uzonna JE, Myal Y. Regulation of immunity in breast cancer. Cancers. 2019;11(8):1080.
Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60.
Nam GH, Choi Y, Kim GB, Kim S, Kim SA, Kim IS. Emerging prospects of exosomes for cancer treatment: from conventional therapy to immunotherapy. Adv Mater. 2020;32(51):2002440.
Hussen BM, Abdullah KH, Abdullah SR, Majeed NM, Mohamadtahr S, Rasul MF, et al. New insights of miRNA molecular mechanisms in breast cancer brain metastasis and therapeutic targets. Noncoding RNA Res. 2023;8(4):645–60.
Wang H, He D, Wan K, Sheng X, Cheng H, Huang J, et al. In situ multiplex detection of serum exosomal microRNAs using an all-in-one biosensor for breast cancer diagnosis. Analyst. 2020;145(9):3289–96.
Yi Y, Wu M, Zeng H, Hu W, Zhao C, **ong M, et al. Tumor-derived exosomal non-coding RNAs: the emerging mechanisms and potential clinical applications in breast Cancer. Front Oncol. 2021;11:738945.
Zhou Q, Wang J, Zhang H, Sun L, Liu J, Meng L, Li J. Tumor-derived exosomes RNA expression profiling identifies the prognosis, immune characteristics, and treatment in HR+/HER2-breast cancer. Aging. 2023;15(16):8471–86.
Zhang J, Shi J, Liu W, Zhang K, Zhao H, Zhang H, Zhang Z. A simple, specific and on-off type MUC1 fluorescence aptasensor based on exosomes for detection of breast cancer. Sens Actuators B. 2018;276:552–9.
Kennedy LB, Salama AK. A review of cancer immunotherapy toxicity. Cancer J Clin. 2020;70(2):86–104.
Xu Z, Zeng S, Gong Z, Yan Y. Exosome-based immunotherapy: a promising approach for cancer treatment. Mol Cancer. 2020;19(1):1–16.
Lipson EJ, Forde PM, Hammers H-J, Emens LA, Taube JM, Topalian SL, editors. Antagonists of PD-1 and PD-L1 in cancer treatment. Seminars in oncology. Elsevier; 2015.
Emens LA, Ascierto PA, Darcy PK, Demaria S, Eggermont AM, Redmond WL, et al. Cancer immunotherapy: opportunities and challenges in the rapidly evolving clinical landscape. Eur J Cancer. 2017;81:116–29.
Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546(7659):498–503.
Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9(8):581–93.
Pitt JM, Charrier M, Viaud S, André F, Besse B, Chaput N, Zitvogel L. Dendritic cell–derived exosomes as immunotherapies in the fight against cancer. J Immunol. 2014;193(3):1006–11.
Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–79.
Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell. 2016;164(6):1226–32.
Kalluri R. The biology and function of exosomes in cancer. J Clin Investig. 2016;126(4):1208–15.
Aslan C, Maralbashi S, Salari F, Kahroba H, Sigaroodi F, Kazemi T, Kharaziha P. Tumor-derived exosomes: implication in angiogenesis and antiangiogenesis cancer therapy. J Cell Physiol. 2019;234(10):16885–903.
Tran T-H, Mattheolabakis G, Aldawsari H, Amiji M. Exosomes as nanocarriers for immunotherapy of cancer and inflammatory diseases. Clin Immunol. 2015;160(1):46–58.
Steinbichler TB, Dudás J, Skvortsov S, Ganswindt U, Riechelmann H. Skvortsova I-I. therapy resistance mediated by exosomes. Mol Cancer. 2019;18(1):1–11.
Pu X, Ma S, Gao Y, Xu T, Chang P, Dong L. Mesenchymal stem cell-derived exosomes: biological function and their therapeutic potential in radiation damage. Cells. 2020;10(1):42.
Klinke DJ, Kulkarni YM, Wu Y, Byrne-Hoffman C. Inferring alterations in cell‐to‐cell communication in HER2 + breast cancer using secretome profiling of three cell models. Biotechnol Bioeng. 2014;111(9):1853–63.
Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M, et al. Potential role of HER2-overexpressing exosomes in countering trastuzumab‐based therapy. J Cell Physiol. 2012;227(2):658–67.
Maas SL, Breakefield XO, Weaver AM. Extracellular vesicles: unique intercellular delivery vehicles. Trends Cell Biol. 2017;27(3):172–88.
Johnstone RM, Adam M, Hammond J, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–20.
Ni Z, Zhou S, Li S, Kuang L, Chen H, Luo X, et al. Exosomes: roles and therapeutic potential in osteoarthritis. Bone Res. 2020;8(1):1–18.
Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protocols cell Biology. 2006;30(1):3. 1–3. 9.
Kumar DN, Chaudhuri A, Aqil F, Dehari D, Munagala R, Singh S, et al. Exosomes as Emerging Drug Delivery and Diagnostic modality for breast Cancer: recent advances in isolation and application. Cancers. 2022;14(6):1435.
Chen H, Wang L, Zeng X, Schwarz H, Nanda HS, Peng X, Zhou Y. Exosomes, a new star for targeted delivery. Front Cell Dev Biology. 2021:2827.
Beach A, Zhang H-G, Ratajczak MZ, Kakar SS. Exosomes: an overview of biogenesis, composition and role in ovarian cancer. J Ovarian Res. 2014;7(1):1–11.
Schmidt O, Teis D. The ESCRT machinery. Curr Biol. 2012;22(4):R116–20.
Colombo M, Moita C, Van Niel G, Kowal J, Vigneron J, Benaroch P, et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J Cell Sci. 2013;126(24):5553–65.
Mercier V, Laporte MH, Destaing O, Blot B, Blouin CM, Pernet-Gallay K, et al. ALG-2 interacting protein-X (Alix) is essential for clathrin-independent endocytosis and signaling. Sci Rep. 2016;6(1):1–15.
Février B, Raposo G. Exosomes: endosomal-derived vesicles ship** extracellular messages. Curr Opin Cell Biol. 2004;16(4):415–21.
Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319(5867):1244–7.
Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977.
Gurunathan S, Kang M-H, Kim J-H. A comprehensive review on factors influences biogenesis, functions, therapeutic and clinical implications of exosomes. Int J Nanomed. 2021;16:1281.
Panigrahi AR, Srinivas L, Panda J. Exosomes: insights and therapeutic applications in cancer. Translational Oncol. 2022;21:101439.
Hong C-S, Sharma P, Yerneni SS, Simms P, Jackson EK, Whiteside TL, Boyiadzis M. Circulating exosomes carrying an immunosuppressive cargo interfere with cellular immunotherapy in acute myeloid leukemia. Sci Rep. 2017;7(1):1–10.
Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W, editors. Exosomes and their roles in immune regulation and cancer. Seminars in cell & developmental biology. Elsevier; 2015.
Barros FM, Carneiro F, Machado JC, Melo SA. Exosomes and immune response in cancer: friends or foes? Front Immunol. 2018;9:730.
Yang Y, Li C-W, Chan L-C, Wei Y, Hsu J-M, **a W, et al. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res. 2018;28(8):862–4.
Lugini L, Cecchetti S, Huber V, Luciani F, Macchia G, Spadaro F, et al. Immune surveillance properties of human NK cell-derived exosomes. J Immunol. 2012;189(6):2833–42.
Huntington ND, Cursons J, Rautela J. The cancer–natural killer cell immunity cycle. Nat Rev Cancer. 2020;20(8):437–54.
Liu J, Ren L, Li S, Li W, Zheng X, Yang Y, et al. The biology, function, and applications of exosomes in cancer. Acta Pharm Sinica B. 2021;11(9):2783–97.
Hegmans JP, Gerber PJ, Lambrecht BN, Exosomes. Functional Proteomics: Springer; 2008. p. 97–109.
Whiteside TL. Immune modulation of T-cell and NK (natural killer) cell activities by TEXs (tumour-derived exosomes). Biochem Soc Trans. 2013;41(1):245–51.
Clayton A, Tabi Z. Exosomes and the MICA-NKG2D system in cancer. Blood Cells Molecules Dis. 2005;34(3):206–13.
Bauernhofer T, Kuss I, Henderson B, Baum AS, Whiteside TL. Preferential apoptosis of CD56dim natural killer cell subset in patients with cancer. Eur J Immunol. 2003;33(1):119–24.
Liu C, Yu S, Zinn K, Wang J, Zhang L, Jia Y, et al. Murine mammary carcinoma exosomes promote tumor growth by suppression of NK cell function. J Immunol. 2006;176(3):1375–85.
Wen SW, Sceneay J, Lima LG, Wong CS, Becker M, Krumeich S, et al. The Biodistribution and Immune Suppressive effects of breast Cancer–derived ExosomesExosomes regulate Immune Composition in Metastatic organs. Cancer Res. 2016;76(23):6816–27.
Xu HY, Li N, Yao N, Xu XF, Wang HX, Liu XY, Zhang Y. CD8 + T cells stimulated by exosomes derived from RenCa cells mediate specific immune responses through the FasL/Fas signaling pathway and, combined with GM–CSF and IL–12, enhance the anti–renal cortical adenocarcinoma effect. Oncol Rep. 2019;42(2):866–79.
Poggio M, Hu T, Pai C-C, Chu B, Belair CD, Chang A, et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177(2):414–27. e13.
Theodoraki M-N, Yerneni SS, Hoffmann TK, Gooding WE, Whiteside TL. Clinical significance of PD-L1 + exosomes in plasma of Head and Neck Cancer PatientsPD-L1 + exosomes in plasma of HNC patients. Clin Cancer Res. 2018;24(4):896–905.
Wang Y, Goliwas KF, Severino PE, Hough KP, Van Vessem D, Wang H, et al. Mechanical strain induces phenotypic changes in breast cancer cells and promotes immunosuppression in the tumor microenvironment. Lab Invest. 2020;100(12):1503–16.
Chatterjee S, Chatterjee A, Jana S, Dey S, Roy H, Das MK, et al. Transforming growth factor beta orchestrates PD-L1 enrichment in tumor-derived exosomes and mediates CD8 T-cell dysfunction regulating early phosphorylation of TCR signalome in breast cancer. Carcinogenesis. 2021;42(1):38–47.
Wada J, Onishi H, Suzuki H, Yamasaki A, Nagai S, Morisaki T, Katano M. Surface-bound TGF-β1 on effusion-derived exosomes participates in maintenance of number and suppressive function of regulatory T-cells in malignant effusions. Anticancer Res. 2010;30(9):3747–57.
Yin Y, Cai X, Chen X, Liang H, Zhang Y, Li J, et al. Tumor-secreted miR-214 induces regulatory T cells: a major link between immune evasion and tumor growth. Cell Res. 2014;24(10):1164–80.
Kalvala A, Wallet P, Yang L, Wang C, Li H, Nam A, et al. Phenotypic switching of naïve T cells to immune-suppressive Treg-like cells by mutant KRAS. J Clin Med. 2019;8(10):1726.
Ni C, Fang Q-Q, Chen W-Z, Jiang J-X, Jiang Z, Ye J, et al. Breast cancer-derived exosomes transmit lncRNA SNHG16 to induce CD73 + γδ1 Treg cells. Signal Transduct Target Therapy. 2020;5(1):41.
Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The nature of myeloid-derived suppressor cells in the tumor microenvironment. Trends Immunol. 2016;37(3):208–20.
Filipazzi P, Bürdek M, Villa A, Rivoltini L, Huber V, editors. Recent advances on the role of tumor exosomes in immunosuppression and disease progression. Seminars in cancer biology. Elsevier; 2012.
Gao Y, Xu H, Li N, Wang H, Ma L, Chen S, et al. Renal cancer-derived exosomes induce tumor immune tolerance by MDSCs-mediated antigen-specific immunosuppression. Cell Communication Signal. 2020;18(1):1–14.
Jiang M, Zhang W, Zhang R, Liu P, Ye Y, Yu W, et al. Cancer exosome-derived miR-9 and miR-181a promote the development of early-stage MDSCs via interfering with SOCS3 and PIAS3 respectively in breast cancer. Oncogene. 2020;39(24):4681–94.
**ang X, Poliakov A, Liu C, Liu Y, Deng Zb, Wang J, et al. Induction of myeloid-derived suppressor cells by tumor exosomes. Int J Cancer. 2009;124(11):2621–33.
Fu H, Yang H, Zhang X, Xu W. The emerging roles of exosomes in tumor–stroma interaction. J Cancer Res Clin Oncol. 2016;142:1897–907.
Yan Z, Sheng Z, Zheng Y, Feng R, **ao Q, Shi L, et al. Cancer-associated fibroblast-derived exosomal miR-18b promotes breast cancer invasion and metastasis by regulating TCEAL7. Cell Death Dis. 2021;12(12):1120.
Lee J-K, Park S-R, Jung B-K, Jeon Y-K, Lee Y-S, Kim M-K, et al. Exosomes derived from mesenchymal stem cells suppress angiogenesis by down-regulating VEGF expression in breast cancer cells. PLoS ONE. 2013;8(12):e84256.
Zhu Y, Tao Z, Chen Y, Lin S, Zhu M, Ji W, et al. Exosomal MMP-1 transfers metastasis potential in triple-negative breast cancer through PAR1-mediated EMT. Breast Cancer Res Treat. 2022;193(1):65–81.
Tung KH, Ernstoff MS, Allen C, Shu S. A review of exosomes and their role in the Tumor Microenvironment and Host-Tumor Macroenvironment. J Immunol Sci. 2019;3(1):4–8.
Yu Dd, Wu Y, Shen Hy L, Mm C, Wx Z, Xh, et al. Exosomes in development, metastasis and drug resistance of breast cancer. Cancer Sci. 2015;106(8):959–64.
György B, Hung ME, Breakefield XO, Leonard JN. Therapeutic applications of extracellular vesicles: clinical promise and open questions. Annu Rev Pharmacol Toxicol. 2015;55:439.
Liang Y, Duan L, Lu J, **a J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11(7):3183.
Huang L, Rong Y, Tang X, Yi K, Qi P, Hou J, et al. Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer. Mol Cancer. 2022;21(1):1–19.
Gong C, Tian J, Wang Z, Gao Y, Wu X, Ding X, et al. Functional exosome-mediated co-delivery of doxorubicin and hydrophobically modified microRNA 159 for triple-negative breast cancer therapy. J Nanobiotechnol. 2019;17(1):1–18.
Yu M, Gai C, Li Z, Ding D, Zheng J, Zhang W, et al. Targeted exosome-encapsulated erastin induced ferroptosis in triple negative breast cancer cells. Cancer Sci. 2019;110(10):3173–82.
Zhao L, Gu C, Gan Y, Shao L, Chen H, Zhu H. Exosome-mediated siRNA delivery to suppress postoperative breast cancer metastasis. J Controlled Release. 2020;318:1–15.
Ohno S-i, Takanashi M, Sudo K, Ueda S, Ishikawa A, Matsuyama N, et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol Ther. 2013;21(1):185–91.
Han D, Wang K, Zhang T, Gao G-C, Xu H. Natural killer cell-derived exosome-entrapped paclitaxel can enhance its anti-tumor effect. Eur Rev Med Pharmacol Sci. 2020;24(10).
Hashemi ZS, Ghavami M, Kiaie SH, Mohammadi F, Barough MS, Khalili S, et al. Novel delivery of sorafenib by natural killer cell-derived exosomes-enhanced apoptosis in triple-negative breast cancer. Nanomedicine. 2023;18(5):437–53.
Wylie B, Macri C, Mintern JD, Waithman JJC. Dendritic cells and cancer: from biology to therapeutic intervention. 2019;11(4):521.
Boer MC, Joosten SA, Ottenhoff THJF. Regulatory T-cells at the interface between human host and pathogens in infectious diseases and vaccination. 2015;6:217.
Admyre C, Johansson SM, Paulie S, Gabrielsson S. Direct exosome stimulation of peripheral humanT cells detected by ELISPOT. Eur J Immunol. 2006;36(7):1772–81.
Vincent-Schneider H, Stumptner‐Cuvelette P, Lankar D, Pain S, Raposo G, Benaroch P, Bonnerot C. Exosomes bearing HLA‐DR1 molecules need dendritic cells to efficiently stimulate specific T cells. Int Immunol. 2002;14(7):713–22.
Romagnoli GG, Zelante BB, Toniolo PA, Migliori IK, Barbuto JAM. Dendritic cell-derived exosomes may be a tool for cancer immunotherapy by converting tumor cells into immunogenic targets. Front Immunol. 2015;5:692.
O’Sullivan TE, Sun JC, Lanier LL. Natural killer cell memory. Immunity. 2015;43(4):634–45.
Di Pace AL, Tumino N, Besi F, Alicata C, Conti LA, Munari E, et al. Characterization of human NK cell-derived exosomes: role of DNAM1 receptor in exosome-mediated cytotoxicity against tumor. Cancers. 2020;12(3):661.
Zhu L, Gangadaran P, Kalimuthu S, Oh JM, Baek SH, Jeong SY, et al. Novel alternatives to extracellular vesicle-based immunotherapy–exosome mimetics derived from natural killer cells. Artif Cells Nanomed Biotechnol. 2018;46(sup3):166–79.
Kaban K, Hinterleitner C, Zhou Y, Salva E, Kantarci AG, Salih HR, Märklin M. Therapeutic silencing of Bcl-2 using Nk cell-derived exosomes as a novel therapeutic approach in breast cancer. Cancers. 2021;13(10):2397.
Borst J, Ahrends T, Bąbała N, Melief CJ, Kastenmüller W. CD4 + T cell help in cancer immunology and immunotherapy. Nat Rev Immunol. 2018;18(10):635–47.
Wang L, **e Y, Ahmed KA, Ahmed S, Sami A, Chibbar R, et al. Exosomal pMHC-I complex targets T cell-based vaccine to directly stimulate CTL responses leading to antitumor immunity in transgenic FVBneuN and HLA-A2/HER2 mice and eradicating trastuzumab-resistant tumor in athymic nude mice. Breast Cancer Res Treat. 2013;140(2):273–84.
Hao S, Liu Y, Yuan J, Zhang X, He T, Wu X, et al. Novel exosome-targeted CD4 + T cell vaccine counteracting CD4 + 25 + regulatory T cell-mediated immune suppression and stimulating efficient central memory CD8 + CTL responses. J Immunol. 2007;179(5):2731–40.
**e Y, Wang L, Freywald A, Qureshi M, Chen Y, **ang J. A novel T cell-based vaccine capable of stimulating long-term functional CTL memory against B16 melanoma via CD40L signaling. Cell Mol Immunol. 2013;10(1):72–7.
Li R, Chibbar R, **ang J. Novel EXO-T vaccine using polyclonal CD4 + T cells armed with HER2-specific exosomes for HER2-positive breast cancer. OncoTargets Therapy. 2018;11:7089.
Shi X, Cheng Q, Hou T, Han M, Smbatyan G, Lang JE, et al. Genetically engineered cell-derived nanoparticles for targeted breast cancer immunotherapy. Mol Ther. 2020;28(2):536–47.
Cheng Q, Shi X, Zhang Y. Reprogramming exosomes for Immunotherapy. Cell Reprogramming for Immunotherapy: Springer; 2020. pp. 197–209.
Cao Y, Rodgers DT, Du J, Ahmad I, Hampton EN, Ma JS, et al. Design of switchable chimeric antigen receptor T cells targeting breast cancer. Angew Chem Int Ed. 2016;55(26):7520–4.
Yang P, Cao X, Cai H, Feng P, Chen X, Zhu Y, et al. The exosomes derived from CAR-T cell efficiently target mesothelin and reduce triple-negative breast cancer growth. Cell Immunol. 2021;360:104262.
Heusinkveld M, van Der Burg SH. Identification and manipulation of tumor associated macrophages in human cancers. J Translational Med. 2011;9:1–14.
Ham S, Lima LG, Chai EPZ, Muller A, Lobb RJ, Krumeich S, et al. Breast cancer-derived exosomes alter macrophage polarization via gp130/STAT3 signaling. Front Immunol. 2018;9:871.
O’Connell RM, Taganov KD, Boldin MP, Cheng G, Baltimore D. MicroRNA-155 is induced during the macrophage inflammatory response. Proceedings of the National Academy of Sciences. 2007;104(5):1604-9.
Chaudhuri AA, So AY-L, Sinha N, Gibson WS, Taganov KD, O’Connell RM, Baltimore D. MicroRNA-125b potentiates macrophage activation. J Immunol. 2011;187(10):5062–8.
Moradi-Chaleshtori M, Bandehpour M, Soudi S, Mohammadi-Yeganeh S, Hashemi SM. In vitro and in vivo evaluation of anti-tumoral effect of M1 phenotype induction in macrophages by miR-130 and miR-33 containing exosomes. Cancer Immunol Immunother. 2021;70:1323–39.
Moradi-Chaleshtori M, Shojaei S, Mohammadi-Yeganeh S, Hashemi SM. Transfer of miRNA in tumor-derived exosomes suppresses breast tumor cell invasion and migration by inducing M1 polarization in macrophages. Life Sci. 2021;282:119800.
Altanerova U, Jakubechova J, Benejova K, Priscakova P, Pesta M, Pitule P, et al. Prodrug suicide gene therapy for cancer targeted intracellular by mesenchymal stem cell exosomes. Int J Cancer. 2019;144(4):897–908.
Lee J, Henderson K, Massidda MW, Armenta-Ochoa M, Im BG, Veith A, et al. Mechanobiological conditioning of mesenchymal stem cells for enhanced vascular regeneration. Nat Biomedical Eng. 2021;5(1):89–102.
Li H, ** Y, Zhao Y, Li W, He Z, Zhang Q, et al. Targeted cell therapy for partial-thickness cartilage defects using membrane modified mesenchymal stem cells by transglutaminase 2. Biomaterials. 2021;275:120994.
Gomari H, Forouzandeh Moghadam M, Soleimani M. Targeted cancer therapy using engineered exosome as a natural drug delivery vehicle. OncoTargets Therapy. 2018:5753–62.
O’brien K, Khan S, Gilligan K, Zafar H, Lalor P, Glynn C, et al. Employing mesenchymal stem cells to support tumor-targeted delivery of extracellular vesicle (EV)-encapsulated microRNA-379. Oncogene. 2018;37(16):2137–49.
Vakhshiteh F, Rahmani S, Ostad SN, Madjd Z, Dinarvand R, Atyabi F. Exosomes derived from miR-34a-overexpressing mesenchymal stem cells inhibit in vitro tumor growth: a new approach for drug delivery. Life Sci. 2021;266:118871.
Naseri Z, Oskuee RK, Forouzandeh-Moghadam M, Jaafari MR. Delivery of LNA-antimiR-142-3p by mesenchymal stem cells-derived exosomes to breast cancer stem cells reduces tumorigenicity. Stem cell Reviews Rep. 2020;16:541–56.
Wang J-H, Forterre AV, Zhao J, Frimannsson DO, Delcayre A, Antes TJ, et al. Anti-HER2 scfv-directed extracellular vesicle-mediated mRNA-based gene delivery inhibits growth of HER2-positive human breast tumor xenografts by prodrug activation. Mol Cancer Ther. 2018;17(5):1133–42.
Forterre AV, Wang J-H, Delcayre A, Kim K, Green C, Pegram MD, et al. Extracellular vesicle–mediated in Vitro transcribed mRNA delivery for treatment of HER2 + breast Cancer xenografts in mice by Prodrug CB1954 without General ToxicitySide Effect–Free GDEPT in mice by CB1954 (Tretazicar). Mol Cancer Ther. 2020;19(3):858–67.
Hadla M, Palazzolo S, Corona G, Caligiuri I, Canzonieri V, Toffoli G, Rizzolio F. Exosomes increase the therapeutic index of doxorubicin in breast and ovarian cancer mouse models. Nanomedicine. 2016;11(18):2431–41.
Tran PH, Wang T, Yin W, Tran TT, Barua HT, Zhang Y, et al. Development of a nanoamorphous exosomal delivery system as an effective biological platform for improved encapsulation of hydrophobic drugs. Int J Pharm. 2019;566:697–707.
Kalimuthu S, Gangadaran P, Rajendran RL, Zhu L, Oh JM, Lee HW, et al. A new approach for loading anticancer drugs into mesenchymal stem cell-derived exosome mimetics for cancer therapy. Front Pharmacol. 2018;9:1116.
Tian Y, Li S, Song J, Ji T, Zhu M, Anderson GJ, et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials. 2014;35(7):2383–90.
Li D, Yao S, Zhou Z, Shi J, Huang Z, Wu Z. Hyaluronan decoration of milk exosomes directs tumor-specific delivery of doxorubicin. Carbohydr Res. 2020;493:108032.
Jung I, Shin S, Baek M-C, Yea K. Modification of immune cell-derived exosomes for enhanced cancer immunotherapy: current advances and therapeutic applications. Exp Mol Med. 2024:1–13.
Yamashita T, Takahashi Y, Takakura Y. Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol Pharm Bull. 2018;41(6):835–42.
Willis GR, Kourembanas S, Mitsialis SA. Toward exosome-based therapeutics: isolation, heterogeneity, and fit-for-purpose potency. Front Cardiovasc Med. 2017;4:63.
Acknowledgements
The authors would like to thank all the staff of the Immunology Research Center of Tabriz University of Medical Sciences for the sincere collaboration and support during the period of conducting this study.
Funding
No funding.
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Safaei, S., Fadaee, M., Farzam, O.R. et al. Exploring the dynamic interplay between exosomes and the immune tumor microenvironment: implications for breast cancer progression and therapeutic strategies. Breast Cancer Res 26, 57 (2024). https://doi.org/10.1186/s13058-024-01810-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13058-024-01810-z