Abstract
Background
Near infrared brain functional imaging (FNIRS) has been used for the evaluation of brain functional areas, the imaging differences of central activation of cognitive-motor dual tasks between patients with chronic lateral ankle instability (CLAI) and healthy population remain unclear. This study aimed to evaluated the role of central imaging based on FNIRS technology on the plan management in patients with CLAI, to provide insights to the clinical treatment of CLAI.
Methods
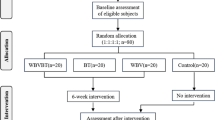
CLAI patients treated in our hospital from January 1, 2021 to June 31, 2022 were selected. Both CLAI patients and health controls were intervened with simple task and cognitive-motor dual task under sitting and walking conditions, and the changes of oxygenated hemoglobin concentration in bilateral prefrontal cortex (PFC), premotor cortex (PMC) and auxiliary motor area (SMA) were collected and compared.
Results
A total of 23 participants were enrolled. There were significant differences in the fNIRS ΔHbO2 of barefoot subtractive walking PFC-R and barefoot subtractive walking SMA-R between experimental and control group (all P < 0.05). There was no significant difference in ΔHbO2 between the experimental group and the control group in other states (P > 0.05). There was no significant difference in ΔHbO2 between the experimental group and the control group in each state of the brain PMC region.
Conclusion
Adaptive alterations may occur within the relevant brain functional regions of individuals with CLAI. The differential activation observed between the PFC and the SMA could represent a compensatory mechanism emerging from proprioceptive afferent disruptions following an initial ankle sprain.
Similar content being viewed by others
Background
Ankle sprain is one of the most common sports injuries at present, especially among the people who participate in competitive sports [1, 2], with a prevalence rate of 23%∼61% [3, 4]. The prevalence rate of external ankle sprain in the general population is also high [5]. Up to 70% of the ordinary people report having ankle injuries in their lifetime [6]. Moreover, the probability of lateral ankle re injury is as high as 80% [7]. Up to 73% of people with ankle sprains will suffer from repeated sprains [8], accompanied by a feeling of instability of the ankle [9]. The interaction of these residual mechanical injuries and sensorimotor injury symptoms promotes the development of chronic lateral ankle instability (CLAI) [10]. The instability of the ankle joint has also become a problem for more and more people [11], and the sprain injury of the lateral ankle joint has formed a higher socio-economic cost [12]. Previous studies have shown that disruptions in proprioceptive afferents of the ankle joint are considered consequences of the initial lateral malleolus sprain and may be contributory to the underlying pathogenesis of chronic ankle instability [13,14,15]. The poor posture and balance control ability of patients with chronic ankle instability during movement output may be related to limited joint activity and insufficient muscle strength [16, 17]. While a multitude of researchers posit that alterations in lower limb kinematics among patients with chronic ankle instability represent an adaptive protective compensation, empirical studies validating the connection between modifications in movement patterns and the presence of ankle instability remain scant [28] has found that the activation of bilateral auxiliary motor areas increased during walking in healthy people, and the activation of PFC, PMC and primary sensorimotor cortex (SMC) increased during walking and running. At the same time, Suzuki et al. [29] has found that PFC plays an important role in the process of adapting to the change of walking speed in healthy people. Bilateral activation of the PFC and PMC exhibits a significant increase during the acceleration phase of walking, with the SMC showing minimal variation in activation. In contrast, the medial PFC demonstrates the most pronounced changes in activation during the running phase. An fMRI study has showed an increase in PFC activation during the walk initiation phase [30] and an increase in SMA activation during the walk initiation phase [31]. Miyai et al. [32] has evaluated the changes of cerebral hemoglobin concentration in 6 stroke patients during walking. Previous study [33] has showed that the activation of frontal lobe area in healthy people decreased after walking acceleration, while the frontal lobe area in stroke patients remained active after walking acceleration. Therefore, the frontal lobe area may be a compensatory area for pace regulation.
Doi et al. [34] have found that PFC activation in the prefrontal lobe increased when the elderly performed cognitive tasks while walking, while Beurskens et al. [35] have found that prefrontal lobe activity decreased significantly when elderly people performed judgment tasks while walking, and there was no change in brain activity when walking and communicating at the same time. Task complexity seems to affect brain activity in the prefrontal lobe of young people during walking. Hill et al. [36] have found that PFC activation increased significantly only when performing more difficult cognitive tasks. At the same time, Beurskens et al. [35] have observed a significant decrease in PFC activation during visual dual tasks, while Holtzer et al. [37] have found that PFC activation increased in young people when performing oral tasks, especially when walking. Previous studies have shown that the minus 7 subtraction task (randomly selected numbers from 200 to 300 minus 7 times in a row) seems to hinder the stable activation of leg muscles in patients with CAI, while verbal memory tasks lead to more protective landing strategies [38]. Therefore, the continuous subtraction 7 subtraction task is enough to affect the functional activity performance of CAI patients. The study employs a continuous subtraction task, specifically subtracting seven repeatedly, as an oral component that mandates participants to announce their calculation outcomes in an ongoing manner. This approach enhances the task’s complexity and facilitates a more distinct observation of brain functional area activation changes in patients with CLAI.
Previous studies [39, 40] have shown that subcortical and subcortical regions are involved in stable bipedal walking, which requires specific motor networks in the brain. The direct pathway guides the movement through the M1 area, cerebellum and spinal cord, while the indirect pathway regulates the movement through the prefrontal cortex, auxiliary motor area and basal ganglia [41]. In addition, the prefrontal cortex is important for top-down regulation [42] and promoting subcortical motor pathway connections [43]. Some activation of the prefrontal cortex and anterior cingulate cortex when walking with specific goals, more complex walking tasks, and dual-task walking. However, the prefrontal cortex is also more active during fast walking [44]. The increase in PFC activity may need to distinguish between related stimuli and irrelevant stimuli. SMA involves non-speed control and exercise plan execution [45], which may be a necessary factor to ensure posture and motor stability during high-speed movement.
Reduced activity in specific brain regions may be a compensatory strategy for older individuals to ensure the activity of a wide range of cortical networks needed to successfully solve tasks [46,47,48]. The overactivity in specific brain regions of patients with nervous system also reflects the compensation strategy described by Stern [49]. The co-activation of multiple sensory areas in the elderly may be a compensatory strategy for peripheral sensory defects. The elderly are more dependent on the co-activation of multiple sensory areas, while the young show a task-oriented activation model [50]. The increase in prefrontal cortex and SMA activity in the elderly [51] also reflects a compensatory strategy. Kluzik et al. [23] have shown that after chronic ankle instability and other musculoskeletal system injuries, the central processing system will also have adaptive changes, in which the reduced functional areas will be dominated by other functional areas, which may be one of the reasons for the kinematic changes of the lower extremities. There is no difference in the change of HbO2 in cerebral cortex between CAI and healthy subjects, but the variability of HbO2 concentration in patients with CAI is greater than that in healthy subjects, indicating that the strategy of cortical activation may be changed to maintain limb balance [52].
Changes in exercise preparation or feedforward exercise plans, and changes in SMA activities indicate that this feedforward control has been affected. Therefore, this change in cortical activation may be an adaptive change that plays a role in successfully coordinating dynamic tasks [53, 54]. Different from the results of previous studies, we have found that there are significant differences in the activity of right PFC and right SMA between the experimental group and the control group during dual tasks. During the dual-task conditions, the experimental group exhibited significantly heightened activity in the right PFC compared to the control group. Conversely, the activation of the SMA on the right side was notably reduced in the experimental group relative to the control group. These findings suggest that adaptive alterations may be occurring within the brain’s functional regions in patients with CLAI. Patients with CLAI need higher attention control and motor control executive function in PFC area. At the same time, the degree of activation of the SMA area, which dominates non-speed control and exercise planning, may be one of the causes of postural control and motor stability disorders, and indirectly lead to the occurrence and development of chronic ankle instability [55, 56].
The fNIRS technology utilized in this study represents a novel method for monitoring brain function, which has been extensively applied in the diagnosis, treatment, and research of cerebral functions, as well as in the monitoring of pain processes [57,60]. There are still some limitations in this study. First, the sample size used in this study is small. Secondly, the cognitive-motor dual task used in this study is the oral subtraction task, and the dual tasks such as image discrimination and continuous conversation are not used for comparison. In addition, our study takes the change of HbO2 concentration as the target parameter, excluding indicators such as HbR and HbT, and does not include parameters such as the slope and peak value of the hemoglobin curve. Therefore, in the future studies, it is necessary to expand the sample size and extract more characteristic parameters to obtain more comprehensive research results.
Conclusions
The results of this study have showed that the PFC of CLAI patients is more active than that of healthy controls, while the activation of SMA area decreases significantly. The increase of PFC activation may be due to the fact that CLAI patients need more attention allocation and motor control functions when performing dual tasks. At the same time, the decrease of SMA activation indicates that the ability of posture control and exercise planning is decreased in CLAI patients, which suggests that adaptive changes may have taken place in the brain functional areas of CLAI patients. Previous studies have also shown that there will be adaptive changes in the central processing system of patients with CAI, and limited brain resources make individuals form compensatory strategies [61, 62]. The disparity in activation between the PFC and the SMA may not solely represent a compensatory strategy emerging from proprioceptive afferent disruptions following an initial ankle sprain. It could also contribute to a diminished capacity for posture control and motor stability, potentially exacerbating the progression towards CLAI. To elucidate the relationship between the activation changes in brain functional areas involved in sensory integration, motor execution, and posture control, and the development of CLAI, further studies with larger participant sample size are warranted. Additionally, investigating the connection between the excitability of sensory and motor conduction tracts of the spinal cord and CLAI could provide valuable insights.
Data availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
- CLAI:
-
chronic lateral ankle instability
- fNIRS:
-
near infrared brain functional imaging
- PFC:
-
bilateral prefrontal cortex
- PMC:
-
premotor cortex
- SMA:
-
auxiliary motor area
- HbO2 :
-
oxygenated hemoglobin
References
Brown CN, Mynark R. Balance deficits in recreational athletes with chronic ankle instability. J Athl Train. 2007;42(3):367–73.
de Azevedo Sodre Silva A, Sassi LB, Martins TB, de Menezes FS, Migliorini F, Maffulli N, Okubo R. Epidemiology of injuries in young volleyball athletes: a systematic review. J Orthop Surg Res. 2023;18(1):748.
Simon J, Hall E, Docherty C. Prevalence of chronic ankle instability and associated symptoms in university dance majors: an exploratory study. J Dance Med Sci. 2014;18(4):178–84.
Tanen L, Docherty CL, Van Der Pol B, Simon J, Schrader J. Prevalence of chronic ankle instability in high school and division I athletes. Foot Ankle Spec. 2014;7(1):37–44.
Feger MA, Glaviano NR, Donovan L, Hart JM, Saliba SA, Park JS, Hertel J. Current trends in the management of lateral ankle sprain in the United States. Clin J Sport Med. 2017;27(2):145–52.
Hiller CE, Nightingale EJ, Raymond J, Kilbreath SL, Burns J, Black DA, Refshauge KM. Prevalence and impact of chronic musculoskeletal ankle disorders in the community. Arch Phys Med Rehabil. 2012;93(10):1801–7.
Freeman MA, Dean MR, Hanham IW. The etiology and prevention of functional instability of the foot. J Bone Joint Surg Br. 1965;47(4):678–85.
Olmsted LC, Carcia CR, Hertel J, Shultz SJ. Efficacy of the Star Excursion Balance tests in detecting Reach deficits in subjects with chronic ankle instability. J Athl Train. 2002;37(4):501–6.
Gribble PA, Delahunt E, Bleakley C, Caulfield B, Docherty CL, Fourchet F, Fong D, Hertel J, Hiller C, Kaminski TW, et al. Selection criteria for patients with chronic ankle instability in controlled research: a position statement of the International Ankle Consortium. J Orthop Sports Phys Ther. 2013;43(8):585–91.
Hertel J. Functional anatomy, Pathomechanics, and pathophysiology of lateral ankle instability. J Athl Train. 2002;37(4):364–75.
Gribble PA, Bleakley CM, Caulfield BM, Docherty CL, Fourchet F, Fong DT, Hertel J, Hiller CE, Kaminski TW, McKeon PO, et al. Evidence review for the 2016 International Ankle Consortium consensus statement on the prevalence, impact and long-term consequences of lateral ankle sprains. Br J Sports Med. 2016;50(24):1496–505.
Delahunt E, Bleakley CM, Bossard DS, Caulfield BM, Docherty CL, Doherty C, Fourchet F, Fong DT, Hertel J, Hiller CE, et al. Clinical assessment of acute lateral ankle sprain injuries (ROAST): 2019 consensus statement and recommendations of the International Ankle Consortium. Br J Sports Med. 2018;52(20):1304–10.
Hertel J. Sensorimotor deficits with ankle sprains and chronic ankle instability. Clin Sports Med. 2008;27(3):353–70. vii.
Freeman MA. Instability of the foot after injuries to the lateral ligament of the ankle. J Bone Joint Surg Br. 1965;47(4):669–77.
Ferran NA, Oliva F, Maffulli N. Ankle instability. Sports Med Arthrosc Rev. 2009;17(2):139–45.
Hoch MC, Staton GS, Medina McKeon JM, Mattacola CG, McKeon PO. Dorsiflexion and dynamic postural control deficits are present in those with chronic ankle instability. J Sci Med Sport. 2012;15(6):574–9.
Son SJ, Kim H, Seeley MK, Hopkins JT. Movement strategies among groups of chronic ankle instability, Coper, and control. Med Sci Sports Exerc. 2017;49(8):1649–61.
**aojian S, Hanjia, Yu L, Xueqiang W, Peijie C. Research progress on pathological mechanism, evaluation and diagnosis of chronic ankle instability. Chin J Sports Med. 2019;11(9):816–24.
Ferran NA, Maffulli N. Epidemiology of sprains of the lateral ankle ligament complex. Foot Ankle Clin. 2006;11(3):659–62.
Baetens T, De Kegel A, Palmans T, Oostra K, Vanderstraeten G, Cambier D. Gait analysis with cognitive-motor dual tasks to distinguish fallers from nonfallers among rehabilitating stroke patients. Arch Phys Med Rehabil. 2013;94(4):680–6.
Mitra S, Knight A, Munn A. Divergent effects of cognitive load on quiet stance and task-linked postural coordination. J Exp Psychol Hum Percept Perform. 2013;39(2):323–8.
Weeks DL, Forget R, Mouchnino L, Gravel D, Bourbonnais D. Interaction between attention demanding motor and cognitive tasks and static postural stability. Gerontology. 2003;49(4):225–32.
Kluzik J, Peterka RJ, Horak FB. Adaptation of postural orientation to changes in surface inclination. Exp Brain Res. 2007;178(1):1–17.
Edwards AD, Wyatt JS, Richardson C, Delpy DT, Cope M, Reynolds EO. Cotside measurement of cerebral blood flow in ill newborn infants by near infrared spectroscopy. Lancet. 1988;2(8614):770–1.
Jalalvandi M, Riyahi Alam N, Sharini H, Hashemi H, Nadimi M. Brain cortical activation during Imagining of the wrist Movement using functional Near-Infrared Spectroscopy (fNIRS). J Biomed Phys Eng. 2021;11(5):583–94.
J M, S H, N Y, RA N. Assessment of Brain cortical activation in Passive Movement during wrist Task using functional Near Infrared Spectroscopy (fNIRS). Front Biomed Technol. 2019;6(2):99–105.
Harada T, Miyai I, Suzuki M, Kubota K. Gait capacity affects cortical activation patterns related to speed control in the elderly. Exp Brain Res. 2009;193(3):445–54.
Miyai I, Tanabe HC, Sase I, Eda H, Oda I, Konishi I, Tsunazawa Y, Suzuki T, Yanagida T, Kubota K. Cortical map** of gait in humans: a near-infrared spectroscopic topography study. NeuroImage. 2001;14(5):1186–92.
Suzuki M, Miyai I, Ono T, Oda I, Konishi I, Kochiyama T, Kubota K. Prefrontal and premotor cortices are involved in adapting walking and running speed on the treadmill: an optical imaging study. NeuroImage. 2004;23(3):1020–6.
Sahyoun C, Floyer-Lea A, Johansen-Berg H, Matthews PM. Towards an understanding of gait control: brain activation during the anticipation, preparation and execution of foot movements. NeuroImage. 2004;21(2):568–75.
Yazawa S, Shibasaki H, Ikeda A, Terada K, Nagamine T, Honda M. Cortical mechanism underlying externally cued gait initiation studied by contingent negative variation. Electroencephalogr Clin Neurophysiol. 1997;105(5):390–9.
Miyai I, Yagura H, Oda I, Konishi I, Eda H, Suzuki T, Kubota K. Premotor cortex is involved in restoration of gait in stroke. Ann Neurol. 2002;52(2):188–94.
Mihara M, Miyai I, Hatakenaka M, Kubota K, Sakoda S. Sustained prefrontal activation during ataxic gait: a compensatory mechanism for ataxic stroke? NeuroImage. 2007;37(4):1338–45.
Doi T, Makizako H, Shimada H, Park H, Tsutsumimoto K, Uemura K, Suzuki T. Brain activation during dual-task walking and executive function among older adults with mild cognitive impairment: a fNIRS study. Aging Clin Exp Res. 2013;25(5):539–44.
Beurskens R, Helmich I, Rein R, Bock O. Age-related changes in prefrontal activity during walking in dual-task situations: a fNIRS study. Int J Psychophysiol. 2014;92(3):122–8.
Hill A, Bohil C, Lewis J, Neider M. Prefrontal cortex activity during walking while multitasking: an fNIR study. Proc Hum. 2013;23(57):1224–8.
Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J. fNIRS study of walking and walking while talking in young and old individuals. J Gerontol Biol Sci Med Sci. 2011;66(8):879–87.
Watson EL, Bearden AC, Doughton JH, Needle AR. The effects of multiple modalities of cognitive loading on dynamic Postural Control in individuals with chronic ankle instability. Gait Posture. 2020;79:10–5.
Hamacher D, Herold F, Wiegel P, Hamacher D, Schega L. Brain activity during walking: a systematic review. Neurosci Biobehav Rev. 2015;57:310–27.
Pelicioni PHS, Tijsma M, Lord SR, Menant J. Prefrontal cortical activation measured by fNIRS during walking: effects of age, disease and secondary task. PeerJ. 2019;7:e6833.
Jacobsen NSJ, Blum S, Scanlon JEM, Witt K, Debener S. Mobile electroencephalography captures differences of walking over even and uneven terrain but not of single and dual-task gait. Front Sports Act Living. 2022;4:945341.
Funahashi S, Andreau JM. Prefrontal cortex and neural mechanisms of executive function. J Physiol Paris. 2013;107(6):471–82.
Rae CL, Hughes LE, Anderson MC, Rowe JB. The prefrontal cortex achieves inhibitory control by facilitating subcortical motor pathway connectivity. J Neurosci. 2015;35(2):786–94.
Belli V, Orcioli-Silva D, Beretta VS, Vitorio R, Zampier VC, Nobrega-Sousa P, Conceicao NRD, Gobbi LTB. Prefrontal cortical activity during Preferred and fast walking in Young and older adults: an fNIRS Study. Neuroscience. 2021;473:81–9.
Fukuyama H, Ouchi Y, Matsuzaki S, Nagahama Y, Yamauchi H, Ogawa M, Kimura J, Shibasaki H. Brain functional activity during gait in normal subjects: a SPECT study. Neurosci Lett. 1997;228(3):183–6.
Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage. 2002;17(3):1394–402.
Heuninckx S, Wenderoth N, Swinnen SP. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci. 2008;28(1):91–9.
Rosano C, Aizenstein H, Cochran J, Saxton J, De Kosky S, Newman AB, Kuller LH, Lopez OL, Carter CS. Functional neuroimaging indicators of successful executive control in the oldest old. NeuroImage. 2005;28(4):881–9.
Stern Y, Barulli D. Cognitive reserve. Handb Clin Neurol. 2019;167:181–90.
van Hedel HJA, Bulloni A, Gut A. Prefrontal cortex and supplementary motor Area Activation during Robot-assisted weight-supported Over-ground walking in Young neurological patients: a Pilot fNIRS Study. Front Rehabil Sci. 2021;2:788087.
Allali G, van der Meulen M, Beauchet O, Rieger SW, Vuilleumier P, Assal F. The neural basis of age-related changes in motor imagery of gait: an fMRI study. J Gerontol Biol Sci Med Sci. 2014;69(11):1389–98.
Cai T, Zhu H, Xu J, Wu S, Li X, He S. Human cortical neural correlates of visual fatigue during binocular depth perception: an fNIRS study. PLoS ONE. 2017;12(2):e0172426.
Rosen AB, Yentes JM, McGrath ML, Maerlender AC, Myers SA, Mukherjee M. Alterations in cortical activation among individuals with chronic ankle instability during single-limb Postural Control. J Athl Train. 2019;54(6):718–26.
Bridgman SA, Clement D, Downing A, Walley G, Phair I, Maffulli N. Population based epidemiology of ankle sprains attending accident and emergency units in the West Midlands of England, and a survey of UK practice for severe ankle sprains. Emerg Med J. 2003;20(6):508–10.
Vitorio R, Stuart S, Rochester L, Alcock L, Pantall A. fNIRS response during walking - artefact or cortical activity? A systematic review. Neurosci Biobehav Rev. 2017;83:160–72.
Putzolu M, Samogin J, Cosentino C, Mezzarobba S, Bonassi G, Lagravinese G, Vato A, Mantini D, Avanzino L, Pelosin E. Neural oscillations during motor imagery of complex gait: an HdEEG study. Sci Rep. 2022;12(1):4314.
M HS. Identification of the Pain process by Cold Stimulation: using Dynamic Causal modeling of effective connectivity in Functional Near-Infrared Spectroscopy (fNIRS). IRBM. 2019;40(2):86–94.
Zhou X, Wan Y, Xu Z, Yu C, Wu Z, Zhuang Z, **a R, Wang H, Chen S. Utilizing fNIRS to investigate the impact of Baduan** training on attentional function in post-stroke cognitive impairment patients: a study protocol for a randomized controlled trial. BMC Complement Med Ther. 2024;24(1):30.
Zu YM, Luo LN, Chen XP, ** of active and passive upper limb training in stroke patients and healthy people: A functional near-infrared spectroscopy study. Brain Research. 2022;1788:147935.
McPhee AM, Cheung TCK, Schmuckler MA. Dual-task interference as a function of varying motor and cognitive demands. Front Psychol. 2022;13:952245.
Sui SX, Hendy AM, Teo WP, Moran JT, Nuzum ND, Pasco JA. A review of the measurement of the neurology of Gait in Cognitive Dysfunction or Dementia, focusing on the application of fNIRS during Dual-Task Gait Assessment. Brain Sci 2022, 12(8).
Acknowledgements
None.
Funding
This study was supported by National Key R&D Program Project (2023YFC3603702), the Shanghai Municipal Health Commission Health Industry Clinical Research Special Project (202240250), China Disabled Persons’ Federation special projects of assistive products for the disabled (2022CDPFAT-15), National Natural Science Foundation of China (32071308) and Shanghai Yangzhi Rehabilitation Hospital (Shanghai Sunshine Rehabilitation Center) hospital-level research project (YJKYQN202014).
Author information
Authors and Affiliations
Contributions
X L, T W and W N designed research; X L, B H, Y H, M L, W N, T W conducted research; X L, B H analyzed data; X L, T W wrote the first draft of manuscript; X L, T W had primary responsibility for final content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was submitted to the Ethics Committee of the Shanghai Yangzhi Rehabilitation Hospital and was approved with approval number SBKT-2022-060(2024-017). All participants were clearly informed of the purpose and process of the experiment and signed the written informed consent form.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Luo, X., Huang, B., Huang, Y. et al. Central imaging based on near-infrared functional imaging technology can be useful to plan management in patients with chronic lateral ankle instability. J Orthop Surg Res 19, 361 (2024). https://doi.org/10.1186/s13018-024-04790-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-024-04790-0