Abstract
Background
Guided bone self-generation with periosteum-preserved has successfully regenerated mandibular, temporomandibular and interphalangeal joint. The aim of this study was to investigate the dynamic changes of gene expression of periosteum which was involved in the guided bone self-generation.
Methods
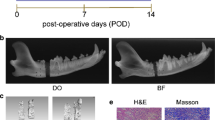
Rib defects of critical size were created in mature swine with periosteum-preserved. The periosteum was sutured into a sealed sheath that closed the bone defect. The periosteum of trauma and control sites were harvested at postoperative 9 time points, and total RNA was extracted. Microarray analysis was conducted to identify the differences in the transcriptome of different time points between two groups.
Results
The differentially expressed genes (DEGs) between control and trauma group were different at postoperative different time points. The dynamic changes of the number of DEGs fluctuated a lot. There were 3 volatility peaks, and we chose 3 time points of DEG number peak (1 week, 5 weeks and 6 months) to study the functions of DEGs. Oxidoreductase activity, oxidation–reduction process and mitochondrion are the most enriched terms of Go analysis. The major signaling pathways of DEGs enrichment include oxidative phosphorylation, PI3K-Akt signaling pathway, osteoclast differentiation pathway and Wnt signaling.
Conclusions
The oxidoreductase reaction was activated during this bone regeneration process. The oxidative phosphorylation, PI3K-Akt signaling pathway, osteoclast differentiation pathway and Wnt signaling may play important roles in the guided bone self-generation with periosteum-preserved. This study can provide a reference for how to improve the application of this concept of bone regeneration.
Similar content being viewed by others
Introduction
Periosteum, a highly vascularized connective tissue, plays a key role in the growth, development and regeneration of bone [39]. Fracture repair was considered to be a recapitulation of embryonic development, and members of the Wnt signaling pathway were activated [40]. Nan et al. found that Wnt signaling pathway was activated during bone regeneration [40]. The Wnt signaling pathway may play an important role in the regenerative process.
Several strategies, including gene therapy and tissue engineering together with mesenchymal stem cells (MSC), have been proposed to promote the healing of the musculoskeletal tissue. Moreover, a recent technology has revolutionized gene editing: Clustering regulatory interval short palindromic repeats (CRISPR) features simple target design, affordable, versatile and efficient, but requires more research to be the preferred platform for genome editing. Predictive genomics DNA analysis can understand which genetic advantages (if any) can be exploited and why specific rehabilitation programs are more effective in some people than others [41, 42]. Therefore, a better understanding of the genetic impact on musculoskeletal system function and disease healing is needed to plan and develop patient-specific management strategies. Currently, while some results are promising, all biological interventions are experimental and the cost/effectiveness has not been proven. In addition, the short follow-up time of most studies questioned the durability of treatment [42]. In this study, autologous periosteum was used to guide bone regeneration in vivo, and the regenerated bone was used for precise repair of the body. This technology has a promising clinical application prospect. The related differential genes and signaling pathways identified in this study can provide a rich theoretical basis for later gene and molecular intervention.
Though this in vivo study provides a better understanding in the molecular mechanisms involved in guided self-generation, it also has limitations. First, micro-CT can be conducted to evaluate the conditions of bone regeneration at different time points. The association of changes of molecules and bone regeneration can be analyzed to provide more precise explanations of the mechanisms. Besides, different parts of the regeneration may be at different stage of healing process. Immuno-histochemistry to localize the transcript and protein expression may provide deeper appreciation of the function of specific genes.
Conclusions
This study shows the guided bone regeneration involves DEGs associated with oxidation–reduction process, mitochondrion and oxidoreductase activity. The main signaling pathways includes oxidative phosphorylation, PI3K-Akt signaling pathway, osteoclast differentiation pathway and Wnt signaling. This study could deepen our understanding of the molecular mechanisms involved in the guided bone regeneration. With these findings of molecular changes at different time points, it gains the potential of regulating the specific mechanism to enhance the bone regeneration.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Fan W, Crawford R, **ao Y. Structural and cellular differences between metaphyseal and diaphyseal periosteum in different aged rats. Bone. 2008;42:81.
Matsushima S, Isogai N, Jacquet R, Lowder E, Tokui T, Landis WJ. The nature and role of periosteum in bone and cartilage regeneration. Cells Tissues Organs. 2011;194:320.
Lin Z, Fateh A, Salem DM, Intini G. Periosteum: biology and applications in craniofacial bone regeneration. J Dent Res. 2014;93:109.
Augustin G, Antabak A, Davila S. The periosteum. Part 1: anatomy, histology and molecular biology. Injury. 2007;38:1115.
Colnot C, Zhang X, Knothe Tate ML. Current insights on the regenerative potential of the periosteum: molecular, cellular, and endogenous engineering approaches. J Orthop Res. 2012;30:1869.
Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 2009;24:274.
Mesgarzadeh AH, Abadi A, Keshani F. Seven-year follow-up of spontaneous bone regeneration following segmental mandibulectomy: alternative option for mandibular reconstruction. Dent Res J. 2019;16:435.
Sharma P, Williams R, Monaghan A. Spontaneous mandibular regeneration: another option for mandibular reconstruction in children. Br J Oral Maxillofac Surg. 2013;51:e63.
Ahmad O, Omami G. Self-regeneration of the mandible following hemimandibulectomy for ameloblastoma: a case report and review of literature. J Maxillofac Oral Surg. 2015;14:245.
Wei J, Herrler T, Han D, et al. Autologous temporomandibular joint reconstruction independent of exogenous additives: a proof-of-concept study for guided self-generation. Sci Rep. 2016;6:37904.
Wei J, Herrler T, Dai C, Liu K, Han D, Li Q. Guided self-generation of vascularized neo-bone for autologous reconstruction of large mandibular defects. J Craniofac Surg. 2016;27:958.
Wei J, Herrler T, Liu K, et al. The role of cell seeding, bioscaffolds, and the in vivo microenvironment in the guided generation of osteochondral composite tissue. Tissue Eng Part A. 2016;22:1337.
Warnke PH, Springer IN, Wiltfang J, et al. Growth and transplantation of a custom vascularised bone graft in a man. Lancet Lond Engl. 2004;364:766.
Xu H, Han D, Dong JS, et al. Rapid prototyped PGA/PLA scaffolds in the reconstruction of mandibular condyle bone defects. Int J Med Robot Comput Assist Surg MRCAS. 2010;6:66.
Camal Ruggieri IN, Cícero AM, Issa J, Feldman S. Bone fracture healing: perspectives according to molecular basis. J Bone Miner Metab. 2021;39:311.
Zhang X, Zhao G, Zhang Y, et al. Activation of JNK signaling in osteoblasts is inversely correlated with collagen synthesis in age-related osteoporosis. Biochem Biophys Res Commun. 2018;504:771.
**e K, Chen L, Yang J. RNA sequencing evidences the prevention of oxidative stress is effective in injury-induced heterotopic ossification treatment. J Biomed Nanotechnol. 2021;17:196.
Nahm KY, Heo JS, Lee JH, et al. Gene profiling of bone around orthodontic mini-implants by RNA-sequencing analysis. Biomed Res Int. 2015;2015:538080.
Vacek TP, Kalani A, Voor MJ, Tyagi SC, Tyagi N. The role of homocysteine in bone remodeling. Clin Chem Lab Med. 2013;51:579.
Levaot N, Hershfinkel M. How cellular Zn(2+) signaling drives physiological functions. Cell Calcium. 2018;75:53.
Malavasi F, Deaglio S, Zaccarello G, et al. The hidden life of NAD+-consuming ectoenzymes in the endocrine system. J Mol Endocrinol. 2010;45:183.
Faulkner G, Lanfranchi G, Valle G. Telethonin and other new proteins of the Z-disc of skeletal muscle. IUBMB Life. 2001;51:275.
Goley ED, Rammohan A, Znameroski EA, Firat-Karalar EN, Sept D, Welch MD. An actin-filament-binding interface on the Arp2/3 complex is critical for nucleation and branch stability. Proc Natl Acad Sci USA. 2010;107:8159.
Lobo JG, Leite AL, Pereira HA, et al. Low-level fluoride exposure increases insulin sensitivity in experimental diabetes. J Dent Res. 2015;94:990.
Chen CT, Shih YR, Kuo TK, Lee OK, Wei YH. Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells (Dayton, Ohio). 2008;26:960.
Varum S, Rodrigues AS, Moura MB, et al. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS ONE. 2011;6:e20914.
Hofmann AD, Beyer M, Krause-Buchholz U, Wobus M, Bornhäuser M, Rödel G. OXPHOS supercomplexes as a hallmark of the mitochondrial phenotype of adipogenic differentiated human MSCs. PLOS ONE. 2012;7:e35160.
Lee AR, Moon DK, Siregar A, et al. Involvement of mitochondrial biogenesis during the differentiation of human periosteum-derived mesenchymal stem cells into adipocytes, chondrocytes and osteocytes. Arch Pharmacal Res. 2019;42:1052.
Brunt KR, Weisel RD, Li RK. Stem cells and regenerative medicine—future perspectives. Can J Physiol Pharmacol. 2012;90:327.
Mara CS, Sartori AR, Duarte AS, Andrade AL, Pedro MA, Coimbra IB. Periosteum as a source of mesenchymal stem cells: the effects of TGF-β3 on chondrogenesis. Clinics (Sao Paulo, Brazil). 2011;66:487.
Murphy MP, Hartley RC. Mitochondria as a therapeutic target for common pathologies. Nat Rev Drug Discov. 2018;17:865.
**ang L, **e G, Ou J, Wei X, Pan F, Liang H. The extra domain A of fibronectin increases VEGF-C expression in colorectal carcinoma involving the PI3K/AKT signaling pathway. PLOS ONE. 2012;7:e35378.
Kita K, Kimura T, Nakamura N, Yoshikawa H, Nakano T. PI3K/Akt signaling as a key regulatory pathway for chondrocyte terminal differentiation. Genes Cells Devot Mol Cell Mech. 2008;13:839.
Li G, Wang L, Jiang Y, et al. Upregulation of Akt signaling enhances femoral fracture healing by accelerating atrophic quadriceps recovery. Biochim Biophys Acta Mol Basis Dis. 2017;186:2848.
Zhang H, Chen X, Xue P, Ma X, Li J, Zhang J. FN1 promotes chondrocyte differentiation and collagen production via TGF-β/PI3K/Akt pathway in mice with femoral fracture. Gene. 2021;769:145253.
Ferretti C, Vozzi G, Falconi M, et al. Role of IGF1 and IGF1/VEGF on human mesenchymal stromal cells in bone healing: two sources and two fates. Tissue Eng Part A. 2014;20:2473.
Sato K, Suematsu A, Nakashima T, et al. Regulation of osteoclast differentiation and function by the CaMK-CREB pathway. Nat Med. 2006;12:1410.
Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40:251.
Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science (New York, NY). 2002;296:1644.
Zhong N, Gersch RP, Hadjiargyrou M. Wnt signaling activation during bone regeneration and the role of Dishevelled in chondrocyte proliferation and differentiation. Bone. 2006;39:5.
Aicale R, Tarantino D, Maccauro G, Peretti GM, Maffulli N. Genetics in orthopaedic practice. J Biol Regul Homeost Agents. 2019;33:103.
Andia I, Maffulli N. New biotechnologies for musculoskeletal injuries. Surgeon. 2019;17:244.
Acknowledgements
None.
Funding
This research was supported by grants from the Shanghai Municipal Key Clinical Specialty (shslczdzk00901 to J.W) and the National Natural Science Foundation of China (No. 81871572).
Author information
Authors and Affiliations
Contributions
DCC and WJ designed the study and supported funding. YBF, WZ and CXX were major contributors in writing and reviewing the manuscript. YBF, WZ, CXX, ZQ and YN completed experiments and analyzed the data. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Consent for publication
All the authors agree to the submission and consent for publication this paper.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1
. Volcano maps of the DEGs between control and trauma group at postoperative different time points (A: 1 day, B: 3 days, C: 1 week, D: 2 weeks, E: 1 month, F: 5 weeks, G: 3 months, H: 6 months, I: 7 months).
Additional file 2: Figure S2
. Results of cluster analysis revealed unknown biological connections between genes through expression clustering.
Additional file 3: Figure S3
. Top 10 GO term entries with the smallest p-value. A, The top 10 GO term entries of postoperative 1 week. B, The top 10 GO term entries of postoperative 5 weeks. C, The top 10 GO term entries of postoperative 6 months.
Additional file 4: Figure S4
. PI3K-Akt signaling pathway.
Additional file 5: Figure S5
. Osteoclast differentiation pathway.
Additional file 6: Figure S6
. Wnt signaling pathway.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yu, BF., Wang, Z., Chen, XX. et al. Continuous dynamic identification of key genes and molecular signaling pathways of periosteum in guided bone self-generation in swine model. J Orthop Surg Res 18, 53 (2023). https://doi.org/10.1186/s13018-023-03524-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13018-023-03524-y