Abstract
Background
Postoperative pain is a serious clinical problem with a poorly understood mechanism, and lacks effective treatment. Hydrogen (H2) can reduce neuroinflammation; therefore, we hypothesize that H2 may alleviate postoperative pain, and aimed to investigate the underlying mechanism.
Methods
Mice were used to establish a postoperative pain model using plantar incision surgery. Mechanical allodynia was measured using the von Frey test. Cell signaling was assayed using gelatin zymography, western blotting, immunohistochemistry, and immunofluorescence staining. Animals or BV-2 cells were received with/without ASK1 and Trx1 inhibitors to investigate the effects of H2 on microglia.
Results
Plantar incision surgery increased MMP-9 activity and ASK1 phosphorylation in the spinal cord of mice. MMP-9 knockout and the ASK1 inhibitor, NQDI-1, attenuated postoperative pain. H2 increased the expression of Trx1 in the spinal cord and in BV-2 cells. H2 treatment mimicked NQDI1 in decreasing the phosphorylation of ASK1, p38 and JNK. It also reduced MMP-9 activity, downregulated pro-IL-1β maturation and IBA-1 expression in the spinal cord of mice, and ameliorated postoperative pain. The protective effects of H2 were abolished by the Trx1 inhibitor, PX12. In vitro, in BV-2 cells, H2 also mimicked NQDI1 in inhibiting the phosphorylation of ASK1, p38, and JNK, and also reduced MMP-9 activity and decreased IBA-1 expression induced by LPS. The Trx1 inhibitor, PX12, abolished the protective effects of H2 in BV-2 cells.
Conclusions
For the first time, the results of our study confirm that H2 can be used as a therapeutic agent to alleviate postoperative pain through the Trx1/ASK1/MMP9 signaling pathway. MMP-9 and ASK1 may be the target molecules for relieving postoperative pain.
Similar content being viewed by others
Background
Postoperative pain is the most common health problem caused by tissue and nerve lesions after surgical injury. Approximately 80% of patients who experience surgical injury have acute pain postoperatively, and this progresses to severe chronic pain in over 20% of patients [1, 2]. However, effective treatments for postoperative pain are still scarce [3,4,5].
Neuroinflammation mediated by glia, especially microglia, induces central sensitization and plays a crucial role in the pathogenesis of pain [4]. Studies have shown that matrix metalloproteinases (MMPs) represent a novel mechanism and potential therapeutic target for neuroinflammation and pain [6,7,8]. The main MMPs involved in the generation and maintenance of pain include MMP-2 and MMP-9. After nerve injury, a rapid and transient increase in MMP-9 in early activated microglia by the cleavage of IL-1β and the activation of p38 induces neuropathic pain, while MMP-2 produces a delayed increase in maintaining neuropathic pain [8, 9]. Further studies have shown that MMP-9 inhibition suppresses astrocyte and microglia activation, and thus alleviates pain, while MMP-9 knockout significantly reduces neuralgia and tolerance to morphine [8,9,10]. Our previous study showed that paeoniflorin inhibits MMP9/2 and suppresses postoperative pain induced by plantar incisions [11]. Therefore, inhibition of MMP9-induced neuroinflammation may be a novel approach for the treatment of postoperative pain.
Results
MMP-9 participates in the development of plantar incision-induced postoperative pain
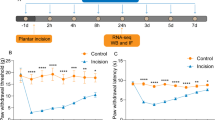
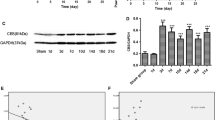
To explore the role of MMP-9 in the process of plantar incision-induced postoperative pain, the activity of MMP-9 and the mechanical threshold of mice (WT or MMP-9−/−) were measured. As shown in Fig. 1A, compared with the sham group, plantar incision surgery significantly decreased the mechanical threshold of the mice for up to 5 days, and caused severe postoperative pain. Plantar incision surgery increased the activity and the expression of MMP-9 in the spinal cord of mice collected 5 days after the operation (Fig. 1B, C). Interestingly, MMP-9 knockout significantly alleviated postoperative pain compare to the WT + PI group (Fig. 1D). These results suggest that plantar incision surgery could be successfully used to establish a postoperative pain model, and that MMP-9 plays a vital role in the development of postoperative pain.
MMP-9 plays a crucial role in the development of plantar incision-induced postoperative pain. A Mechanical threshold of mice that received plantar incision surgery (n = 12). B MMP-9 activity was measured using zymography after surgery in the spinal cord of mice (n = 4). C MMP-9 expression in the spinal cord of mice was measured using western blot after surgery (n = 4). Lumbar spines (L4–L5) were collected and analyzed 5 days after surgery. D Mechanical threshold of wild-type or MMP-9 KO mice that underwent plantar incision surgery (n = 6). PI = plantar incision. Significant difference is revealed following two-way ANOVA (A and D) or unpaired Student’s t-test (B, C) (*P < 0.05, **P < 0.01, ***P < 0.001 vs. sham; &P < 0.05, &&P < 0.01 vs. PI group; Bonferroni post hoc tests)
ASK1 mediates MMP-9 activation and facilitates the development of postoperative pain
Considering the important role of ASK1 in inflammation and apoptosis [29]. Our previous study showed that plantar incisions could increase MMP-9 activity, activate microglia, and promote the development of postoperative pain [11]. In this study, we further showed the importance of MMP-9 in the development of postoperative pain using MMP-9 knockout mice (Fig. 1D). Furthermore, MMP-9 co-localized with microglia IBA-1 and aggravated the cleavage of IL-1β (Fig. 4A). We also found that inhibition of ASK1 could ameliorate pain and reduce MMP-9 activity (Fig. 2), suggesting that MMP-9 and its upstream ASK1 are associated with the facilitation of postoperative pain. Regulation of ASK1/MMP9 in microglia may receive considerable attention as a potential therapeutic target for ameliorating postoperative pain.
HRS and H2 have been confirmed to alleviate hyperpathia and activate autophagy in neuropathic pain models [24, 30]. In this study, HRS was used for the first time to treat postoperative pain. In accordance with the effects of H2 on p-ASK1, p-p38, and p-JNK (Fig. 3A-C), H2 decreased MMP-9 activity and expression, inhibited the expression of IL-1β, IL-6, and TNF-α (Fig. 3D-I), and effectively attenuated incision-induced postoperative pain (Fig. 3J).
Furthermore, we explore how H2 regulates the phosphorylation of ASK1, p38, and JNK. Trx1 is an endogenous 12 kDa multifunctional protein with two redox-active half-cysteine residues -Cys-Gly-Pro-Cys- [31]. It has been identified in all living cells and is related to cell proliferation and apoptosis, where it is responsible for protecting cells from oxidative stress by scavenging ROS [31, 32]. Experimental results show that intravenous administration of recombinant human thioredoxin and overexpression of Trx1 in transgenic mice confer resistance to ROS-induced cell death, ultimately decreasing brain damage in cerebral ischemia models [33, 34]. Trx1 overexpression extends antioxidant protection, attenuates mitochondrial damage, and prolongs survival during sepsis [35]. Furthermore, along with the decrease in Trx1 level, NLRP3 expression increases inflammation in injured tissue [36]. Since Trx1 exerts its role by interacting with its binding protein, ASK1, it inhibits the activation of ASK1 [14]; moreover, our data showed that H2 could increase the expression of Trx1 (Fig. 5A, D, E) and attenuate postoperative pain, which was abolished by the Trx1 inhibitor PX12 (Fig. 5G), these data indicate that Trx1 is an endogenous neuroprotective protein that is involved in proves through which H2 reduces postoperative pain. To further investigate the protection effects of H2, we collected and analyzed BV-2 cells after H2 treatment. We found that H2 could mimic the ASK1 inhibitor NQDI1 as it decreases the phosphorylation of ASK1, p38, and JNK, and reduces MMP-9 activity and expression (Fig. 6A–F). We also found that the protective effects of H2 were abolished by the Trx1 inhibitor, PX12, in BV-2 cells (Fig. 6G–N). These data demonstrate that H2 could attenuate postoperative pain by regulating the Trx1/ASK1/MMP-9 signaling pathway, and provides further details regarding the mechanism of H2 therapy.
H2 has been shown to be safe with few adverse effects. Compared to vitamin E and superoxide dismutase, H2 is a selective antioxidant that reduces cytotoxic oxygen radicals [18]. Inhalation of H2, drinking hydrogen water, injection of hydrogen saline, and direct incorporation of molecular hydrogen by diffusion, including eye drops, baths, and cosmetics, are the main methods of ingesting or consuming H2 [19]. Inhalation of H2 suppresses not only the initial brain injury, but also its progressive damage [18]. H2 has beneficial effects including the promotion of microglia M2 polarization and the reduction of inflammation [20, 23]. Interestingly, oral administration of hydrogen water was reported to alleviate neuropathic pain in mice by reducing oxidative stress. Since oxidative stress injury is an important pathological mechanism of postoperative pain [37], oral administration of hydrogen water may also be useful in attenuating postoperative pain. The oral pathway is more conducive to the promotion of the clinical application of H2.
One of the limitations of the research is that we did not consider gender differences in H2 therapy. We only considered the response of male mice to postoperative pain, while ignoring the difference in magnetic responses to pain. We will explore this in future research.
Conclusions
In summary, we demonstrated that H2 attenuates postoperative pain induced by plantar incisions by inhibiting the ASK1/JNK/p38/MMP9 signaling pathway and microglial activation, and this may be related to Trx1 (Fig. 7). Our findings suggest that H2 may be a potential drug for the treatment of postoperative pain.
H2 protects against plantar incision-induced postoperative pain by upregulating the Trx1/ASK1/MMP-9 signaling pathway. Plantar incision surgery increases ASK1/JNK/p38 phosphorylation mediated microglia activation, enhances MMP-9 activity and IL-1β cleavage, and subsequently induces neuroinflammation, which contributes to the progression of postoperative pain in mice. Inhalation of H2 and administration of HRS, which increases Trx1 expression, could ameliorate postoperative pain
Availability of data and materials
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- MMPs:
-
Matrix metalloproteases
- ASK1:
-
Apoptosis signal-regulated kinase 1
- IL-1β:
-
Interleukin-1β
- TNF-α:
-
Tumor necrosis factor alpha
- ERK:
-
Extracellular signal-regulated kinase
- JNK:
-
C-Jun N-terminal kinase
- Trx1:
-
Thioredoxin
- •OH:
-
Hydroxyl radical
- ONOO-:
-
Peroxynitrite
- HRS:
-
Hydrogen-rich saline
- H2:
-
Hydrogen gas
- LPS:
-
Lipopolysaccharide
- DMSO:
-
Dimethyl sulfoxide
- IBA-1:
-
Anti-ionized calcium-binding adaptor protein 1
- NLRP3:
-
Nod-like receptor family pyrin domain containing protein 3
References
Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, Carter T, Cassidy CL, Chittenden EH, Degenhardt E, et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17:131–57.
Glare P, Aubrey KR, Myles PS. Transition from acute to chronic pain after surgery. Lancet. 2019;393:1537–46.
Wu CL, Raja SN. Treatment of acute postoperative pain. Lancet. 2011;377:2215–25.
Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–84.
Colvin LA, Bull F, Hales TG. Perioperative opioid analgesia-when is enough too much? A review of opioid-induced tolerance and hyperalgesia. Lancet. 2019;393:1558–68.
Kuhad A, Singh P, Chopra K. Matrix metalloproteinases: potential therapeutic target for diabetic neuropathic pain. Expert Opin Ther Targets. 2015;19:177–85.
Liou JT, Sum DC, Liu FC, Mao CC, Lai YS, Day YJ. Spatial and temporal analysis of nociception-related spinal cord matrix metalloproteinase expression in a murine neuropathic pain model. J Chin Med Assoc. 2013;76:201–10.
Kawasaki Y, Xu ZZ, Wang X, Park JY, Zhuang ZY, Tan PH, Gao YJ, Roy K, Corfas G, Lo EH, Ji RR. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med. 2008;14:331–6.
Ji RR, Xu ZZ, Wang X, Lo EH. Matrix metalloprotease regulation of neuropathic pain. Trends Pharmacol Sci. 2009;30:336–40.
Liu WT, Han Y, Liu YP, Song AA, Barnes B, Song XJ. Spinal matrix metalloproteinase-9 contributes to physical dependence on morphine in mice. J Neurosci. 2010;30:7613–23.
Fan YX, Hu L, Zhu SH, Han Y, Liu WT, Yang YJ, Li QP. Paeoniflorin attenuates postoperative pain by suppressing Matrix Metalloproteinase-9/2 in mice. Eur J Pain. 2018;22:272–81.
Cao Y, Li K, Fu KY, **e QF, Chiang CY, Sessle BJ. Central sensitization and MAPKs are involved in occlusal interference-induced facial pain in rats. J Pain. 2013;14:793–807.
Liu MG, Wang RR, Chen XF, Zhang FK, Cui XY, Chen J. Differential roles of ERK, JNK and p38 MAPK in pain-related spatial and temporal enhancement of synaptic responses in the hippocampal formation of rats: multi-electrode array recordings. Brain Res. 2011;1382:57–69.
Liu Y, Min W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ Res. 2002;90:1259–66.
Kosek D, Kylarova S, Psenakova K, Rezabkova L, Herman P, Vecer J, Obsilova V, Obsil T. Biophysical and structural characterization of the thioredoxin-binding domain of protein kinase ASK1 and its interaction with reduced thioredoxin. J Biol Chem. 2014;289:24463–74.
Li Q, Hu L, Li J, Yu P, Hu F, Wan B, Xu M, Cheng H, Yu W, Jiang L, et al. Hydrogen attenuates endotoxin-induced lung injury by activating thioredoxin 1 and decreasing tissue factor expression. Front Immunol. 2021;12: 625957.
Chen CH, Manaenko A, Zhan Y, Liu WW, Ostrowki RP, Tang J, Zhang JH. Hydrogen gas reduced acute hyperglycemia-enhanced hemorrhagic transformation in a focal ischemia rat model. Neuroscience. 2010;169:402–14.
Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–94.
Luchting B, Rachinger-Adam B, Heyn J, Hinske LC, Kreth S, Azad SC. Anti-inflammatory T-cell shift in neuropathic pain. J Neuroinflammation. 2015;12:12.
Chen HG, Han HZ, Li Y, Yu YH, **e KL. Hydrogen alleviated organ injury and dysfunction in sepsis: the role of cross-talk between autophagy and endoplasmic reticulum stress: experimental research. Int Immunopharmacol. 2020;78: 106049.
Yao W, Guo A, Han X, Wu S, Chen C, Luo C, Li H, Li S, Hei Z. Aerosol inhalation of a hydrogen-rich solution restored septic renal function. Aging (Albany NY). 2019;11:12097–113.
Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64:493–502.
Chu X, Cao L, Yu Z, **n D, Li T, Ma W, Zhou X, Chen W, Liu D, Wang Z. Hydrogen-rich saline promotes microglia M2 polarization and complement-mediated synapse loss to restore behavioral deficits following hypoxia-ischemic in neonatal mice via AMPK activation. J Neuroinflammation. 2019;16:104.
Chen H, Zhou C, **e K, Meng X, Wang Y, Yu Y. Hydrogen-rich saline alleviated the hyperpathia and microglia activation via autophagy mediated inflammasome inactivation in neuropathic pain rats. Neuroscience. 2019;421:17–30.
Inoue K, Tsuda M. Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci. 2018;19:138–52.
Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, Smith JW, Liddington RC, Lipton SA. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297:1186–90.
Li J, Xu L, Deng X, Jiang C, Pan C, Chen L, Han Y, Dai W, Hu L, Zhang G, et al. N-acetyl-cysteine attenuates neuropathic pain by suppressing matrix metalloproteinases. Pain. 2016;157:1711–23.
Bordt EA, Polster BM. NADPH oxidase- and mitochondria-derived reactive oxygen species in proinflammatory microglial activation: a bipartisan affair? Free Radic Biol Med. 2014;76:34–46.
Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlap** role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–94.
Wang H, Huo X, Chen H, Li B, Liu J, Ma W, Wang X, **e K, Yu Y, Shi K. Hydrogen-rich saline activated autophagy via HIF-1α pathways in neuropathic pain model. Biomed Res Int. 2018;2018:4670834.
Kondo N, Nakamura H, Masutani H, Yodoi J. Redox regulation of human thioredoxin network. Antioxid Redox Signal. 2006;8:1881–90.
Jia JJ, Geng WS, Wang ZQ, Chen L, Zeng XS. The role of thioredoxin system in cancer: strategy for cancer therapy. Cancer Chemother Pharmacol. 2019;84:453–70.
Hattori I, Takagi Y, Nakamura H, Nozaki K, Bai J, Kondo N, Sugino T, Nishimura M, Hashimoto N, Yodoi J. Intravenous administration of thioredoxin decreases brain damage following transient focal cerebral ischemia in mice. Antioxid Redox Signal. 2004;6:81–7.
Zhou F, Gomi M, Fujimoto M, Hayase M, Marumo T, Masutani H, Yodoi J, Hashimoto N, Nozaki K, Takagi Y. Attenuation of neuronal degeneration in thioredoxin-1 overexpressing mice after mild focal ischemia. Brain Res. 2009;1272:62–70.
Sánchez-Villamil JP, D’Annunzio V, Finocchietto P, Holod S, Rebagliati I, Pérez H, Peralta JG, Gelpi RJ, Poderoso JJ, Carreras MC. Cardiac-specific overexpression of thioredoxin 1 attenuates mitochondrial and myocardial dysfunction in septic mice. Int J Biochem Cell Biol. 2016;81:323–34.
** Y, Li C, Xu D, Zhu J, Wei S, Zhong A, Sheng M, Duarte S, Coito AJ, Busuttil RW, et al. Jagged1-mediated myeloid Notch1 signaling activates HSF1/Snail and controls NLRP3 inflammasome activation in liver inflammatory injury. Cell Mol Immunol. 2020;17:1245–56.
Suter M, Bollen Pinto B, Belletti A, Putzu A. Efficacy and safety of perioperative vitamin C in patients undergoing noncardiac surgery: a systematic review and meta-analysis of randomised trials. Br J Anaesth. 2022;128:664–78.
Acknowledgements
We thank the Nan**g Medical University animal center for providing experimental animals, the Key Laboratory of Neurodegeneration of Nan**g Medical University, and the Central Laboratory of Nan**g Jangning Hospital for providing experimental places and basic materials. We gratefully acknowledge the help of Pan Yu and the Shenzheng Kelieng Hydrogen Molecular Biomedical Institute for assistance with hydrogen generator and hydrogen cell incubator, gas chromatography ENH-1000 and Methylene Blue.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 81971047, 82204542). The Key Research and Development Plan of Jiangsu province (BE2019732). General Projects of Traditional Chinese Medicine Administration of Jiangsu Province (YB201821). Natural Science Research Project of Universities in Jiangsu Province (21KJB310019). Jiangsu Province Hospital (the First Affiliated Hospital with Nan**g Medical University) Clinical Capacity Enhancement Project (JSPH-511B-2018–8). Nan**g special fund for health science and technology development (YKK19170).
Author information
Authors and Affiliations
Contributions
LH, LZ, WL and MD designed and performed the experiments, analyzed the results, and drafted the manuscript. PT, YS and JL carried out the behavioral measures, gelatin zymography and immunofluorescence. QL and LZ carried out the western blotting analysis. WL, MD, PY and LH conceived the study, participated in its design and coordination, and helped to draft the manuscript. JJ, SR, LZ, LH and YP carried out the additional experiments in the revised manuscript and helped to revise the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
All procedures were performed in accordance with the regulations of the ethics committee of the International Association for the Study of Pain and the Guide for the Care and Use of Laboratory Animals (Ministry of Science and Technology of China). All animal experiments were designed to minimize suffering and the number of animals used.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, J., Ruan, S., Jia, J. et al. Hydrogen attenuates postoperative pain through Trx1/ASK1/MMP9 signaling pathway. J Neuroinflammation 20, 22 (2023). https://doi.org/10.1186/s12974-022-02670-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12974-022-02670-0