Abstract
Background
An imbalance between circulating neuroprotective and neurotoxic T cell subsets leads to poor prognosis in acute ischaemic stroke (AIS). Preclinical studies have indicated that the soluble form of the interleukin-2 receptor α (sIL-2Rα)-IL-2 complex regulates T cell differentiation. However, the association between sIL-2Rα levels and AIS remains unclear.
Methods
A total of 201 first-ever AIS patients within 24 h after stroke onset and 76 control subjects were recruited. The National Institutes of Health Stroke Scale (NIHSS) score and 3-month functional outcome (modified Rankin Scale [mRS] score) at admission were assessed. Plasma sIL-2Rα and IL-2 levels at admission were measured. Prognostic significance was identified by using univariate and multivariate logistic regression analyses.
Results
Patients with poor functional outcomes at 3 months had significantly higher levels of sIL-2Rα and lower levels of IL-2 than patients with good outcomes. Moreover, sIL-2Rα levels showed a strong positive correlation with NIHSS and mRS scores (p < 0.0001), whereas IL-2 levels were negatively correlated with mRS scores (p < 0.01). Univariate analyses showed that higher sIL-2Rα and IL-2 levels were associated with an increased and reduced risk of unfavourable outcomes, respectively. After adjusting for confounding variables, the sIL-2Rα level remained independently associated with an increased risk of an unfavourable outcome, and adding sIL-2Rα levels to the conventional risk factor model significantly improved risk reclassification (net reclassification improvement 17.56%, p = 0.003; integrated discrimination improvement 5.78%, p = 0.0003).
Conclusions
sIL-2Rα levels represent a novel, independent prognostic marker that can improve the currently used risk stratification of AIS patients. Our findings also highlight that elevated plasma sIL-2Rα and IL-2 levels manifested opposite correlations with functional outcome, underlining the importance of IL-2/IL-2R autocrine loops in AIS.
Similar content being viewed by others
Background
Stroke is a major cause of global disease burden with limited therapies [1]. One of the many challenges is to identify a cost-effective diagnostic or prognostic biomarker for acute ischaemic stroke (AIS). Increasing evidence has pointed out that an imbalance between circulating neuroprotective and neurotoxic T lymphocyte subsets, such as regulatory T (Treg) and T-helper 17 (Th17) cells, may contribute to poor prognosis in AIS patients [2, 3]. Although various strategies to expand the number of Tregs and inhibit the activation of effector T cells have yielded successful results in preclinical studies [4, 5], the cause of the imbalance in human T cells after AIS has not been well investigated.
Interleukin-2 (IL-2) functions as a multilineage lymphocyte growth factor to promote the proliferation and differentiation of CD4+ T cells into Th1, Th2, Th17 and Treg subsets by binding to low-, medium- or high-affinity receptors (IL-2Rα/IL-2Rβ/γc). In recent years, IL-2 was shown to have therapeutic efficacy by expanding distinct T cell compartments, since low-dose IL-2 preferentially expands Tregs versus other immune cells [6], and some Treg-biased anti-IL-2 antibodies (IL-2Ab) have been developed as therapies for immune diseases [7]. Subsequent work has validated the therapeutic applications of IL-2/IL-2Ab in preclinical models of cerebral ischaemia by inducing Treg proliferation [4]. Although IL-2 is one of the most frequently studied cytokines in AIS patients, the conclusions are ambiguous and inconsistent with the results of experimental studies due to the extensive functionality of IL-2. Thus, more detailed insight is needed into clinically feasible immune therapy targeting the IL-2/IL-2R system in AIS patients.
Elevated plasma levels of the soluble form of interleukin-2 receptor α (sIL-2Rα) reflect the inflammatory process with enhanced T cell activation. Elevated serum levels of sIL-2Rα have been correlated with a poor prognosis in a variety of different types of cancers, immune diseases, and cardiovascular events, including stroke and mortality [8,9,10]. By binding to IL-2, sIL-2Rα upregulates Foxp3 expression and induces the development of regulatory T (Treg) cells [11]. However, its biological relevance in clinical studies remains unclear and controversial. In addition, the association between the plasma level of sIL-2Rα and ischaemic stroke outcome has not been investigated. Therefore, we undertook the present study to explore the clinical significance of sIL-2Rα levels in AIS compared to those of IL-2 to provide new insight into the potential function of IL-2/IL-2R autocrine loops in AIS patients.
Methods
Study participants
The data that support the findings of this study are available from the corresponding author upon reasonable request. We retrospectively screened a consecutive range of patients who were diagnosed with first-ever AIS and presented to Xuanwu Hospital of Capital Medical University within 24 h after symptom onset between November 2018 and May 2019. Our study was approved by the Ethics Committee of Xuanwu Hospital, Capital Medical University. All patients or their immediate family members provided written informed consent. The inclusion criteria were (1) focal or global neurological deficits, (2) a diagnosis of AIS confirmed by brain magnetic resonance imaging (MRI) or computed tomography (CT), (3) no history of stroke, and (4) no pre-stroke disability. Patients with cerebral haemorrhage within the previous 3 months, cancer, rheumatic heart disease, heart failure, renal failure, liver cirrhosis, immune diseases, active infection, epilepsy or other neurological diseases were excluded from our study. Finally, 201 AIS patients were enrolled in our analysis (Fig. 1). Healthy controls were age- and sex-matched relatives of the AIS patients, and those who were examined by experienced neurologists and found to be free of cerebrovascular diseases for more than 12 months were recruited.
Clinical data and blood collection
Baseline data, including demographic characteristics, onset time, blood pressure, comorbidities, and routine laboratory determinations (neutrophil number, lymphocyte number, plasma glucose levels, blood lipids, etc.) at admission were collected from all study participants. Recanalization treatment included recombinant tissue-type plasminogen activator treatment and endovascular treatment. Stroke severity was assessed using the National Institutes of Health Stroke Scale (NIHSS) at admission by trained neurologists. Clinical outcome was evaluated 90 days after stroke by experienced neurologists who were blinded to the biomarker levels using the modified Rankin Scale (mRS). We defined a favourable outcome as a mRS score of 0–2 and an unfavourable outcome as a mRS score of 3–6. Blood samples were collected into K3 EDTA tubes from each AIS patient and healthy control before any treatment. All plasma samples were separated and frozen at − 80 °C.
Measurements of plasma sIL-2Rα and IL-2 levels
Plasma levels of sIL-2Rα and IL-2 were measured in 201 AIS patients and 76 controls using ProcartaPlex™ Multiplex Immunoassay (eBioscience) according to the manufacturer’s instructions [12]. Supernatant samples were then thawed and clarified by centrifugation at 10,000 × g for 10 min. Clarified samples were stored on ice until they were loaded onto plates (∼1 h). Wash buffer (10×) was diluted in deionised water to make a 1× solution, and 1 vial of lyophilized Standard Mix containing all 10 proteins was reconstituted with 250 μL of the same media used to generate the samples. The standard vial was vortexed briefly, centrifuged gently for 10 s and stored on ice for 10 min. A 1:4 serial dilution was performed for a 7-pt standard curve with varying S1–S7 concentrations for each target (see Table 1) and a background tube. Bead Mix (1×) was vortexed for 30 s, and 50 μL was added to each assay well for standards, background and samples. For the wash steps, a magnetic plate separator (Thermo Fisher Scientific Cat. No. EPX-55555-000) was used. Beads were allowed to settle for 2 min, and then the liquid was decanted with a manual pipette. Then, 150 μL of wash buffer was added to each well with beads and allowed to incubate for an additional 15–30 s. The liquid was decanted, and the plate was blotted gently on absorbent paper to remove excess wash buffer. Then, the plate was removed from the magnetic separator. Fifty microlitres of standards, background and samples were run in duplicate. The plate was sealed, and a plate cover was added to protect the plate from light. The plate was loaded onto a small diameter (< 5 mm) plate shaker for 1 min at 800 revolutions per minute (rpm) and then adjusted to 600 rpm for 2 h at room temperature. Wash steps were performed as described above. After the wash steps, 25 μL of detection antibody (1×) was added to each well. The plate was sealed, covered and shaken for 30 min at room temperature. Wash steps were performed as described above. After the wash steps, 50 μL of streptavidin-PE was added to each assay well. The plate was sealed, covered and shaken for 30 min at room temperature. Wash steps were performed as described above. After the wash step, 120 μL of Reading Buffer was added to each assay well. The plate was sealed, covered and shaken at 800 rpm for 5 min at room temperature. Then, the plate cover and seal were removed, and the plate was loaded into the Luminex 200 (LX200) system for reading, which took approximately 15 min. The LX200 with xPonent® 3.1 was allowed to warm for a minimum of 30 min. Calibration and verification steps were performed per the manufacturer’s recommendations. The xPonent software was programmed using the settings from the assay protocol and certificate of analysis. These assays were performed by experienced laboratory technicians blinded to the treatments and conditions.
Statistical analysis
Data were analysed with SPSS 21.0 software (IBM Corp., Armonk, NY, USA) and R software (version 3.5.1). Statistical significance was set at p < 0.05. Continuous variables with a normal distribution were expressed as the means ± SDs and analysed using Student’s t test. Non-normally distributed variables were expressed as medians with interquartile ranges (IQRs) and analysed using the Mann-Whitney U test. For correlation analyses, the Spearman correlation coefficient (rho) was calculated. The chi-squared test was used to compare the frequencies and percentages of categorical variables. Univariate and multivariable logistic regression analyses were used to analyse the relationship between cytokines and the 3-month functional outcomes of AIS patients. The crude and adjusted odds ratios (ORs) and 95% confidence intervals (CIs) of each biomarker were calculated. The multivariable logistic regression analysis included variables that either were previously reported or associated with stroke outcomes in the univariate logistic analyses (p < 0.10). Furthermore, we calculated the net reclassification improvement (NRI) and integrated discrimination improvement (IDI) to quantify the improvement in the correct reclassification and sensitivity with the addition of plasma sIL-2Rα levels to the established risk model according to previous literature [13].
Results
Elevated sIL-2Rα levels were correlated with an increased risk of adverse outcomes of AIS
Plasma sIL-2Rα levels were significantly increased in AIS patients compared to control subjects (p < 0.05, Fig. 2a, Table 1), and a subgroup analysis of patients based on favourable (mRS score 0-2) and unfavourable prognosis (mRS score 3-6) revealed that sIL-2Rα levels were markedly higher in AIS patients with adverse outcomes than in those with good outcomes (p < 0.0001) or control subjects (p < 0.0001, Fig. 2b, Table 1). Furthermore, correlation analysis showed that sIL-2Rα levels were prominently positively correlated with the NIHSS score at admission (p < 0.0001, Fig. 2c) and the mRS score at 3 months post-stroke (p < 0.0001, Fig. 2d) and were significantly negatively correlated with the lymphocyte/neutrophil ratio (p < 0.01, Fig. 2e). Baseline characteristics of the 201 AIS patients stratified by functional outcome (mRS score) at 3 months are shown (Table 2). Poor prognosis was found in 71 (35.32%) patients, with 50 of them (70.42%) being male, and mainly occurred in older patients and patients with high NIHSS scores and glucose levels, a history of atrial fibrillation, high neutrophil-to-lymphocyte ratios and low triglyceride and total cholesterol levels.
Changes in plasma levels of sIL-2Rα and IL-2 in AIS patients and their correlation with neurological deficits. a Plasma level of sIL-2Rα detected by ProcartaPlex in AIS patients and control subjects. b Plasma level of sIL-2Rα in AIS patients with favourable (mRS score = 0–2) and unfavourable (mRS score = 3–6) functional outcomes. c–e Correlation of admission NIHSS score, 3-month mRS score and lymphocyte/neutrophil ratio with the plasma level of sIL-2Rα. f Plasma levels of IL-2 detected by ProcartaPlex in AIS patients and control subjects. g Plasma levels of IL-2 in AIS patients with favourable (mRS score = 0–2) and unfavourable (mRS score = 3–6) functional outcomes. h–j Correlation of admission NIHSS score, 3-month mRS score and lymphocyte/neutrophil ratio with the plasma level of IL-2; N = 76 for controls, N = 201 for AIS patients (N = 128 for the favourable outcome group, N = 73 for the unfavourable outcome group). ****p < 0.0001, ***p < 0.001, *p < 0.05. AIS, acute ischaemic stroke; sIL-2Rα, soluble interleukin-2 receptor α; IL-2, interleukin-2; NIHSS, National Institutes of Health Stroke Scale; mRS, modified Rankin Scale
Increased IL-2 levels were correlated with a reduced risk of unfavourable outcomes of AIS
Plasma IL-2 levels were also markedly upregulated in AIS patients compared to control subjects (p < 0.01, Fig. 2f, Table 1). Subgroup analysis revealed that in contrast to sIL-2Rα levels, IL-2 levels were higher in AIS patients with favourable outcomes than in those with unfavourable outcomes (p < 0.05) or control subjects (p < 0.001, Fig. 2g). Moreover, IL-2 levels showed no correlation with NIHSS scores (Fig. 2h) or lymphocyte/neutrophil ratios (Fig. 2j) but were negatively correlated with mRS scores at 3 months post-stroke (p < 0.05, Fig. 2i).
sIL-2Rα levels represented an independent predictor for unfavourable outcomes of AIS
We further examined the predictive value of sIL-2Rα and IL-2 levels for unfavourable outcomes after AIS and found that in the univariate analyses, high levels of sIL-2Rα were associated with an increased risk of unfavourable outcomes (p < 0.0001, Table 2), while high levels of IL-2 were associated with a decreased risk of unfavourable outcomes (p < 0.05, Table 2).
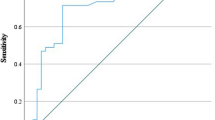
After adjusting for age, admission NIHSS score, a history of diabetes mellitus, coronary heart disease and other variables in the binominal multivariate logistic analysis (model 2), sIL-2Rα levels remained significant for the prediction of unfavourable outcome in AIS patients (p < 0.0001, Table 3). The multivariable adjusted OR (95% CIs) for sIL-2Rα levels (each 100 pg/ml increase) was 1.197 (1.085–1.322). Based on the receiver operating characteristic curve, the optimal cut-off value of the plasma sIL-2Rα level as an indicator for unfavourable outcome was 971.44 pg/ml, yielding a sensitivity of 74.6% and a specificity of 66.9%, with an area under the curve of 0.760. Then, sIL-2Rα levels were dichotomized using the cut-off point identified by the receiver operating characteristic curve. Consistent with the result that the sIL-2Rα level was regarded as a continuous variable, high sIL-2Rα levels (sIL-2Rα ≥ 971.44 pg/ml) remained associated with an increased risk of poor outcome at 3 months after AIS (OR, 5.577; 95% CI, 2.264–13.740). However, we did not find that IL-2 levels were independently associated with unfavourable outcomes after adjusting for confounding variables (Table 3).
Incremental predictive value of sIL-2Rα levels for AIS
We further investigated whether the addition of plasma sIL-2Rα levels to a conventional model with established risk factors could improve its predictive power for unfavourable outcomes (Table 4). The results showed that the addition of plasma sIL-2Rα levels to the conventional model improved the NRI by 17.56% (p = 0.003) and the IDI by 5.78% (p = 0.0003) for the prediction of unfavourable outcomes.
Discussion
We found for the first time that increased levels of sIL-2Rα might be associated with unfavourable outcomes at 3 months after AIS. Particularly, the addition of plasma sIL-2Rα levels to a conventional model with established risk factors substantially improved the risk stratification for primary outcomes. In addition, elevated plasma sIL-2Rα and IL-2 were positively and negatively associated with unfavourable functional outcomes in AIS patients, respectively, indicating that sIL-2Rα and IL-2 might play antagonistic roles in AIS development and that sIL-2Rα is a more important therapeutic target for AIS.
Stroke has high mortality and morbidity. Moreover, biomarkers are required to predict stroke outcomes, which could help clinicians provide rational approaches for patient management. To date, a number of studies have demonstrated that the blood levels of cytokines, chemokines, and growth factors, including C-reactive protein, TNF-α, IL-6 and IL-10, are associated with outcomes in AIS patients [14,15,16]. Although IL-2 is one of the most frequently studied cytokines in stroke patients, the conclusions are ambiguous and inconsistent with the results of experimental studies [17,18,19]. This might be due to the complexity of IL-2/IL-2R autocrine loops. IL-2R is composed of IL-2Rα (CD25), IL-2Rβ (CD122) and IL-2Rγ (CD132) subunits and exists as either αβγ heterotrimeric or βγ heterodimeric complexes. When T cells are activated, the IL2-Rα subunit is released into the blood and becomes sIL-2Rα. It has been reported that sIL-2Rα levels were significantly increased in ischaemic left ventricular dysfunction patients [20, 21] and were positively associated with internal carotid wall thickness and cardiovascular disease mortality [10]. Similarly, we found for the first time that plasma sIL-2Rα levels were significantly increased in AIS patients within 24 h after stroke attack and that sIL-2Rα levels were markedly higher in AIS patients with adverse outcomes than in those with favourable outcomes. In contrast to sIL-2Rα levels, IL-2 levels were higher in AIS patients with favourable outcomes than in those with poor outcomes. Furthermore, univariate analyses confirmed this conclusion, demonstrating that elevated plasma sIL-2Rα and IL-2 levels were associated with an increased and decreased risk of unfavourable outcomes at 3 months, respectively. However, after adjusting for potential contributing factors in the binominal multivariate analysis, we showed that sIL-2Rα levels represented an independent biomarker for predicting functional outcomes in AIS patients and were superior to IL-2 levels. Furthermore, the addition of plasma sIL-2Rα levels to conventional risk factors was shown to improve risk predictions for the primary outcome. We first showed that plasma sIL-2Rα levels might represent a new independent biomarker in AIS risk stratification, which could be beneficial for selecting high-risk patients to receive more aggressive monitoring and therapeutic interventions in future clinical practice.
In numerous clinical observations, the proportion of circulating immune cells is critical to the neurological prognosis of AIS patients, and a low ratio of lymphocytes was shown to predict poor functional outcomes after AIS [22]. We noticed a significant negative correlation between sIL-2Rα levels and lymphocyte/neutrophil ratios, indicating that sIL-2Rα levels might affect the proliferation of human lymphocytes after AIS. With the exception of total lymphocytes, the imbalance between circulating neuroprotective and neurotoxic T lymphocyte subsets also affects prognosis in AIS. Decreasing circulating Tregs and increasing Th17 has been shown to contribute to poor prognosis in AIS [23,24,25,26,27,28,29]. IL-2 functions as a multilineage lymphocyte growth factor to promote the proliferation and differentiation of naive CD4+ T cells. The IL2-Rα subunit is constitutively expressed at high levels on regulatory T (Treg) cells and at lower levels on natural killer cells and resting effector CD8+ T cells, resulting in differential IL-2 potency between the different immune cell compartments. However, when T cells are activated, the IL2-Rα subunit is released into the blood and becomes sIL-2Rα. Preclinical studies indicated that sIL-2Rα competes with IL-2 for binding to IL-2R at the T cell surface and disturbs T cell differentiation [30]. According to our data, increased sIL-2Rα and IL-2 levels were associated with an increased and reduced risk of unfavourable outcomes, respectively. Thus, we speculate that abnormal upregulation of sIL-2Rα is involved in the imbalance between neuroprotective CD4+ T cell subsets (Th2 and Treg) and neurotoxic CD4+ T cell subsets (Th1 and Th17) and promotes neuroinflammation and poor prognosis after AIS. Above all, it is of interest to further elucidate the mechanisms by which the IL2-Rα subunit is released into the blood and increased sIL-2Rα or IL-2/IL-2Rα autocrine loops affect the proportion of CD4+ T cell subsets in AIS.
Conclusions
Our findings provide important clues for both clinical application and basic study. Plasma sIL-2Rα levels represent a novel, independent prognostic marker that can improve the currently used risk stratification in AIS patients within 24 h after stroke attack. Therefore, a combination of plasma sIL-2Rα level determination in conjunction with clinical and imaging examinations will yield the greatest accuracy for predicting a poor outcome for AIS. Further studies with larger sample sizes are needed to verify our findings, and further studies including patients with pre-stroke disability as well as a second or subsequent stroke are needed to determine whether sIL-2Rα levels can predict stroke outcome in a broader AIS patient population. The present study also revealed insights into the initial inflammatory response to ischaemic stroke within 24 h and recognised the potential pathogenic function of IL-2/IL-2R autocrine loops in T lymphocyte differentiation after AIS.
Availability of data and materials
The datasets used during the current study are available from the corresponding author on reasonable request.
Abbreviations
- IL-2:
-
Interleukin-2
- sIL-2Rα:
-
Soluble form of interleukin-2 receptor α
- mRS:
-
Modified Rankin Scale
- NIHSS:
-
National Institutes of Health Stroke Scale
- IDI:
-
Integrated discrimination improvement
- NRI:
-
Net reclassification improvement
References
Bundy DT, Nudo RJ. Preclinical studies of neuroplasticity following experimental brain injury. Stroke. 2019;50:2626–33.
Santamaria-Cadavid M, Rodriguez-Castro E, Rodriguez-Yanez M, Arias-Rivas S, Lopez-Dequidt I, Perez-Mato M, Rodriguez-Perez M, Lopez-Loureiro I, Hervella P, Campos F, et al. Regulatory T cells participate in the recovery of ischemic stroke patients. BMC Neurol. 2020;20:68.
Noh MY, Lee WM, Lee SJ, Kim HY, Kim SH, Kim YS. Regulatory T cells increase after treatment with poly (ADP-ribose) polymerase-1 inhibitor in ischemic stroke patients. Int Immunopharmacol. 2018;60:104–10.
Zhang H, **a Y, Ye Q, Yu F, Zhu W, Li P, Wei Z, Yang Y, Shi Y, Thomson AW, et al. In vivo expansion of regulatory T cells with IL-2/IL-2 antibody complex protects against transient ischemic stroke. J Neurosci. 2018;38:10168–79.
Liu X, Hu R, Pei L, Si P, Wang C, Tian X, Wang X, Liu H, Wang B, **a Z, et al. Regulatory T cell is critical for interleukin-33-mediated neuroprotection against stroke. Exp Neurol. 2020;328:113233.
Matsuoka KI. Low-dose interleukin-2 as a modulator of Treg homeostasis after HSCT: current understanding and future perspectives. Int J Hematol. 2018;107:130–7.
Kohler C, Smole U, Kratzer B, Trapin D, Schmetterer KG, Pickl WF. Allergen alters IL-2/alphaIL-2-based Treg expansion but not tolerance induction in an allergen-specific mouse model. Allergy. 2020.
Zhong H, Chen J, Cheng S, Chen S, Shen R, Shi Q, Xu P, Huang H, Zhang M, Wang L, et al. Prognostic nomogram incorporating inflammatory cytokines for overall survival in patients with aggressive non-Hodgkin’s lymphoma. EBioMedicine. 2019;41:167–74.
Katsuya H, Shimokawa M, Ishitsuka K, Kawai K, Amano M, Utsunomiya A, Hino R, Hanada S, Jo T, Tsukasaki K, et al. Prognostic index for chronic- and smoldering-type adult T-cell leukemia-lymphoma. Blood. 2017;130:39–47.
Durda P, Sabourin J, Lange EM, Nalls MA, Mychaleckyj JC, Jenny NS, Li J, Walston J, Harris TB, Psaty BM, et al. Plasma levels of soluble interleukin-2 receptor alpha: associations with clinical cardiovascular events and genome-wide association scan. Arterioscler Thromb Vasc Biol. 2015;35:2246–53.
Yang ZZ, Grote DM, Ziesmer SC, Manske MK, Witzig TE, Novak AJ, Ansell SM. Soluble IL-2Ralpha facilitates IL-2-mediated immune responses and predicts reduced survival in follicular B-cell non-Hodgkin lymphoma. Blood. 2011;118:2809–20.
Cook DB, McLucas BC, Montoya LA, Brotski CM, Das S, Miholits M, Sebata TH. Multiplexing protein and gene level measurements on a single Luminex platform. Methods. 2019;158:27–32.
Soriano-Tarraga C, Mola-Caminal M, Giralt-Steinhauer E, Ois A, Rodriguez-Campello A, Cuadrado-Godia E, Gomez-Gonzalez A, Vivanco-Hidalgo RM, Fernandez-Cadenas I, Cullell N, et al. Biological age is better than chronological as predictor of 3-month outcome in ischemic stroke. Neurology. 2017;89:830–6.
Rivera-Caravaca JM, Marin F, Vilchez JA, Galvez J, Esteve-Pastor MA, Vicente V, Lip GYH, Roldan V. Refining stroke and bleeding prediction in atrial fibrillation by adding consecutive biomarkers to clinical risk scores. Stroke. 2019;50:1372–9.
Gasbarrino K, Hafiane A, Zheng H, Daskalopoulou SS. Intensive statin therapy compromises the adiponectin-adipoR pathway in the human monocyte-macrophage lineage. Stroke. 2019;50:3609–17.
Nam KW, Kim TJ, Lee JS, Kwon HM, Lee YS, Ko SB, Yoon BW. High neutrophil-to-lymphocyte ratio predicts stroke-associated pneumonia. Stroke. 2018;49:1886–92.
Lasek-Bal A, Jedrzejowska-Szypulka H, Student S, Warsz-Wianecka A, Zareba K, Puz P, Bal W, Pawletko K, Lewin-Kowalik J. The importance of selected markers of inflammation and blood-brain barrier damage for short-term ischemic stroke prognosis. J Physiol Pharmacol. 2019;70.
Cichon N, Saluk-Bijak J, Miller E, Sliwinski T, Synowiec E, Wigner P, Bijak M. Evaluation of the effects of extremely low frequency electromagnetic field on the levels of some inflammatory cytokines in post-stroke patients. J Rehabil Med. 2019;51:854–60.
Martha SR, Cheng Q, Fraser JF, Gong L, Collier LA, Davis SM, Lukins D, Alhajeri A, Grupke S, Pennypacker KR. Expression of cytokines and chemokines as predictors of stroke outcomes in acute ischemic stroke. Front Neurol. 2019;10:1391.
Abbate A, Vecile E, Fiotti N, Giansante C, Guarnieri G, Di Sciascio G, Dobrina A. Plasma concentrations of interleukin-2 soluble receptor in mild ischaemic left ventricular dysfunction. Eur J Heart Fail. 2003;5:23–5.
Limas CJ, Hasikidis C, Iakovou J, Kroupis C, Haidaroglou A, Cokkinos DV. Prognostic significance of soluble interleukin-2 receptor levels in patients with dilated cardiomyopathy. Eur J Clin Investig. 2003;33:443–8.
Pikija S, Sztriha LK, Killer-Oberpfalzer M, Weymayr F, Hecker C, Ramesmayer C, Hauer L, Sellner J. Neutrophil to lymphocyte ratio predicts intracranial hemorrhage after endovascular thrombectomy in acute ischemic stroke. J Neuroinflammation. 2018;15:319.
Lux D, Alakbarzade V, Bridge L, Clark CN, Clarke B, Zhang L, Khan U, Pereira AC. The association of neutrophil-lymphocyte ratio and lymphocyte-monocyte ratio with 3-month clinical outcome after mechanical thrombectomy following stroke. J Neuroinflammation. 2020;17:60.
Xu JH, He XW, Li Q, Liu JR, Zhuang MT, Huang FF, Bao GS. Higher platelet-to-lymphocyte ratio is associated with worse outcomes after intravenous thrombolysis in acute ischaemic stroke. Front Neurol. 2019;10:1192.
Mao L, Li P, Zhu W, Cai W, Liu Z, Wang Y, Luo W, Stetler RA, Leak RK, Yu W, et al. Regulatory T cells ameliorate tissue plasminogen activator-induced brain haemorrhage after stroke. Brain. 2017;140:1914–31.
Albertsson AM, Zhang X, Vontell R, Bi D, Bronson RT, Supramaniam V, Baburamani AA, Hua S, Nazmi A. Cardell S, et al: gammadelta T cells contribute to injury in the develo** brain. Am J Pathol. 2018;188:757–67.
** WN, Gonzales R, Feng Y, Wood K, Chai Z, Dong JF, La Cava A, Shi FD, Liu Q. Brain ischemia induces diversified neuroantigen-specific T-cell responses that exacerbate brain injury. Stroke. 2018;49:1471–8.
Wang X, Zhou Y, Tang D, Zhu Z, Li Y, Huang T, Muller R, Yu W, Li P. ACC1 (acetyl coenzyme A carboxylase 1) is a potential immune modulatory target of cerebral ischemic stroke. Stroke. 2019;50:1869–78.
Zhao H, Li G, Wang R, Tao Z, Ma Q, Zhang S, Han Z, Yan F, Li F, Liu P, et al. Silencing of microRNA-494 inhibits the neurotoxic Th1 shift via regulating HDAC2-STAT4 cascade in ischaemic stroke. Br J Pharmacol. 2020;177:128–44.
Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38:13–25.
Acknowledgements
Not applicable.
Funding
This project was supported by the National Natural Science Foundation of China (81771412, 81771413, and 81971222) and the Bei**g Natural Science Foundation Program and Scientific Research Key Program of the Bei**g Municipal Commission of Education (no. KZ201810025041).
Author information
Authors and Affiliations
Contributions
HPZ and FFL prepared the study protocol; collected, analysed, and interpreted the data; and prepared the manuscript. YYH, SJZ, LZL, ZHY, RLW, ZT, ZPH, JFF, and YMZ collected the data. HPZ and FFL performed the cytometric assay and analysed the data. QFM and YML prepared the study protocol, analysed and interpreted the data, and supervised the study. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Written informed consent was obtained from each patient included in the study. The study protocol was approved by the Xuanwu Hospital of Capital Medical University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhao, H., Li, F., Huang, Y. et al. Prognostic significance of plasma IL-2 and sIL-2Rα in patients with first-ever ischaemic stroke. J Neuroinflammation 17, 237 (2020). https://doi.org/10.1186/s12974-020-01920-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12974-020-01920-3