Abstract
Cholangiocarcinoma (CCA) refers to an aggressive malignancy with a high fatality rate and poor prognosis. Globally, the morbidity of CCA is increasing for the past few decades, which has progressed into a disease that gravely endangers human health. Exosomes belong to a class of extracellular vesicles (EVs) with diameters ranging from 40 to 150 nm that can be discharged by all living cells. As communication messengers of the intercellular network, exosomes carry a diverse range of cargoes such as proteins, nucleic acids, lipids, and metabolic substances, which are capable of conveying biological information across different cell types to mediate various physiological activities or pathological changes. Increasing studies have demonstrated that exosomes in the tumor microenvironment participate in regulating tumorigenesis and progression via multiple approaches in the tumor microenvironment. Here, we reviewed the current research progress of exosomes in the context of cancer and particularly highlighted their functions in modulating the development of CCA. Furthermore, the potential values of exosomes as diagnostic and therapeutic targets in CCA were overviewed as well.
Similar content being viewed by others
Background
CCA remains a highly lethal malignancy of the biliary system. It can be classified into three major groups based on their lesion locations: intrahepatic, perihilar, and distal CCA. Globally, the proportion of CCA is second only to hepatocellular carcinoma (HCC) among all primary liver tumors, accounting for roughly 15%, and accounts for 3% of all gastrointestinal malignant tumors [1, 2]. Despite advancements advances in CCA cognition, diagnosis, and treatment for the past few years, due to its high malignant degree, strong invasiveness, and the occult in initial, most patients are first diagnosed already in the late-stage that severely constrains therapeutic options. Although curative surgery is a preferred selection for some early-stage patients, the fact of tumor recurrence or metastasis after resection remains frustrating. Meanwhile, on account of the high heterogeneity of CCAs, the systemic therapeutics bring little effect for advanced patients who are not possible for radical surgery, leading to a very poor prognosis that only 7%-20% of patients can reach the five-year survival [3, 4]. Nevertheless, with the advancements in genetic profiling of CCAs, emerging treatments such as targeted or immunological therapeutics may be able to assist patients with this deadly cancer to get better outcomes. Considering the current situation of CCAs, exploring new diagnostic and therapeutic strategies remains a matter of priority.
EVs are enveloped by a lipid bilayer that can be discharged by numerous cell sorts. According to the size and formation pathway of vesicles, it can be classified into two major subsets approximately, called ectosomes and exosomes [5]. The former are vesicles with a diameter of 50 nm ~ 1 μm via plasma membrane budding outward directly, while exosomes are EVs ranging from 40 ~ 150 nm in diameter generated in the opposite way, which involves plasma membrane invagination and endosomal formation [5]. Exosomes contain multiple substances and are broadly distributed in different body fluids like plasma, urine, bile, and cerebrospinal fluid (CSF), which play important roles in a variety of normal or abnormal biological behaviors [6,7,8]. Recently, researches about cancer exosomes have received tremendous attention. Intercellular communication in the microenvironment plays a significant role in regulating tumor development, where exosomes are key messengers that mediate this cell-to-cell communication [5, 9]. Previous researches have illustrated that exosomes participate in tumorigenesis or metastasis in multiple ways, their potential usages in cancer diagnosis and prognosis have also been deeply explored [9]. Although certain studies have reviewed the roles of EVs in the progression of CCA [10], a more comprehensive summarization of exosomes in CCA remains insufficient up to now.
In this article, we systematically summarized the research status of exosomes in the tumor fields. Based on the existing researches of exosomes in CCA, we specifically emphasized their significant roles in regulating tumor development and potential values in diagnosis and treatment.
Research status of exosomes
Biogenesis, secretion and internalization
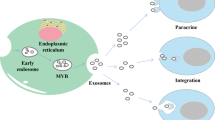
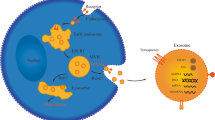
As a type of EVs, the synthesis progress of exosomes involves three major phases: 1) plasma membrane invagination and early endosomes formation. 2) intraluminal vesicles (ILVs) and intracellular multivesicular bodies (MVBs) generation. 3) the fusion of MVBs and plasma membrane leads to exosomes secretion [9]. Generally, the biogenesis of MVBs mainly depends on the following two pathways: endosomal sorting complexes required for transport (ESCRT)-dependent or ESCRT-independent mechanisms, and the former is the most classic pathway [6]. Once mature, MVBs can integrate with autophagosomes then degrade through the lysosomal pathway or secrete into extracellular space as exosomes by fusing with the plasma membrane [11]. In this biogenesis and secretion process, other components such as tumor susceptibility gene 101 (TSG101), Rab family of GTPases (like Rab27A and Rab27B), soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complexes, apoptosis-linked gene 2-interacting protein X (Alix), ceramide, tetraspanins (CD63, CD9, CD81), and phospholipids are also getting involved [5, 12, 13].
Considering the difference in the origin and microenvironment, exosomes have a strong heterogeneity, which is mainly reflected in the regulation of the target cell functions [5]. Once exosomes are secreted by the host cells, they can be absorbed by target cells through various approaches like endocytosis, plasma membrane integration, and specific protein interactions [14]. Among these internalization ways, endocytosis is the most widely studied pattern. According to the characteristics of components involved in endocytosis, several subtypes are broadly divided up, including phagocytosis, macro-pinocytosis, clathrin-mediated endocytosis (CME), and caveolin-dependent endocytosis (CDE), as well as lipid raft-mediated internalization [15]. Moreover, several proteins such as tetraspanins, integrins, proteoglycans, and lectins, also participate in the internalization of exosomes by the unique ligand-receptor interactions[9]. However, on account of the heterogeneity of exosomes, whether or not exosomes uptake is specific remains controversial(15). Therefore, it is essential to further investigate the detailed routes of exosomes uptake (Fig. 1).
Biogenesis, secretion, and internalization of exosomes. The formation of exosomes initially depends on the invagination of the plasma membrane, followed by the generation of ILVs and MVBs. Once mature, MVBs can fuse with lysosomes and be degraded, or integrate with the plasma membrane and finally get released, i.e., exosomes. During this process of synthesis and secretion, ESCRT-dependent and ESCRT-independent mechanisms are two common approaches, other components like the Rab family of GTPases, SNARE, ceramide, and tetraspanins are also involved. Exosomes can be uptake by receptor cells to perform specific functions through various mechanisms, such as phagocytosis, macro-pinocytosis, ligand-receptor interaction, CME, and CDE. As plasma membrane-derived vesicles with lipid bilayer structure, exosomes carry a variety of components, including RNAs (mRNA, MiRNA, LncRNA, and CircRNA), proteins (TSG101, Alix, HSP, CD9, CD63, and CD81) and metabolites, etc.
Isolation and identification
Currently, frequently-used isolation strategies include centrifugation (differential or density gradient centrifugation), particle size separation, size-exclusion chromatography, microfluidic technique, and immunoaffinity capture [16]. Until now, the most common method is still differential centrifugation due to its high exosome yields and relatively cheap price. However, it also has some deficiencies like complicated procedures, low separation efficiency, and susceptibility to contamination by soluble substances in cell culture medium or other body fluids [17]. Other isolation methods like size-exclusion chromatography, with a relatively high yield but difficult to achieve mass production, and immunoaffinity capture, advanced in specific separation yet costly with low yields [16, 17]. So far there is not a standardized method that can achieve both economic and high purity at the same time. Therefore, the exploration of better purification methods remains a major challenge in the exosome-related fields.
In terms of identification, the International Society of Extracellular Vesicles proposed to identify exosomes mainly from the following three aspects: 1) Exosomal morphology identification, 2) Exosomal size detection, 3) Exosomal biomarkers identification [18, 19]. Among them, transmission electron microscopy (TEM), cryo-electron microscopy (Cryo-EM), and atomic force microscopy (AFM) are the most direct methods for visual observation of exosomes [20]. Real-time nanoparticle tracking technology based on the principle of Brownian motion can be used to obtain the size distribution of exosomes [21]. In addition, enzyme-linked immunosorbent assay (ELISA), flow cytometry (FCM), and western blotting (WB) are available means to detect the specific proteins or other markers expressed on exosomes [20, 22]. Reportedly, several transmembrane proteins like CD9, CD63, and CD81 are considered to be representative hallmarks, however, a recent study suggested that compared with other tetraspanins, CD63 is the unique biomarker, while CD9 and CD81 are not specific for exosomes [23]. Moreover, other components related to the formation of exosomes such as Alix, TSG101, and Heat shock proteins (HSP) can also serve as classical hallmarks [5].
The roles of exosomes in malignancies
Since exosomes have played an essential role in multiple pathological changes through mediating intercellular communication, it has also received enormous concerns in the cancer area over the past few years [9]. Related studies have pointed out that cancer-cell-derived exosomes can modulate tumor progression through a variety of mechanisms [9, 24]. Besides, as mentioned above, exosomes contain complex cargoes that are widespread in various body fluids, which also partly represent the heterogeneity of their parental cells, making them available for cancer diagnosis and prognostic by serving as novel biomarkers [25]. Moreover, recent studies have focused more on the tumor microenvironment (TME), where the signal interaction mediated by exosomes also makes a difference in tumor development [5, 26].
Exosomes induce or accelerate tumorigenesis. Exosomes secreted by HCC cancer cells promoted tumorigenesis through the Hedgehog pathway [27]. Mirna-224-5p-enriched exosomes secreted by non-small cell lung cancer (NSCLC) cells accelerated neoplasia by directly binding with the androgen receptor (AR) [28]. On the contrary, exosomes distributed in the plasma of patients with medulloblastoma inhibited tumorigenesis by targeting FOXP4 (forkhead box protein 4) and EZH2 (enhancer of zeste 2 polycomb repressive complex 2 subunit) directly through their miRNA cargoes [ Not applicable. Cholangiocarcinoma Extracellular vesicles Hepatocellular carcinoma Cerebrospinal fluid Intraluminal vesicles Multivesicular bodies Endosomal sorting complexes required for transport Tumor susceptibility gene 101 Soluble N-ethylmaleimide-sensitive factor attachment protein receptor Apoptosis-linked gene 2-interacting protein X Clathrin-mediated endocytosis Caveolin-dependent endocytosis Transmission electron microscopy Cryo-electron microscopy Atomic force microscopy Enzyme-linked immunosorbent assay Flow cytometry Western blotting Heat shock protein MicroRNA Long noncoding RNA Circular RNA Non-small cell lung cancer Androgen receptor Forkhead box protein 4 Enhancer of zeste 2 polycomb repressive complex 2 subunit Phosphatase and tensin homolog Colorectal cancer Kruppel like factor 2 Kruppel like factor 4 Soluble E-cadherin Tumor microenvironment Cancer-associated fibroblasts Epithelial ovarian cancer Epithelial-mesenchymal transition Glycogen synthase kinase 3 beta Oral squamous cell carcinoma Matrix metalloproteinase 9 Colony stimulating factor 1 Monocyte chemoattractant protein-1 Transforming growth factor beta Ubiquitin-like with PHD and ring finger domain 1 Natural killer cells T cell immunoglobulin domain and mucin domain 3 Dendritic cells α-Fetoprotein Programmed death 1 ligand Programmed death 1 Small nucleolar RNA host gene 7 Lung adenocarcinoma Tumor-associated macrophage Autophagy related 5 Glypican-1 Kyoto encyclopedia of genes and genomes Bone marrow mesenchymal stem cells Alpha-smooth muscle actin Fibroblast activation protein alpha Interleukin- 6 Signal transducer and activator of transcription 3 Lymphocyte antigen 6 family member E Cholangiocarcinoma-associated circular RNA 1 Yin Yang 1 Calcium modulating ligand Frizzled class receptor 10 Cytokine-induced killer cells Tumor necrosis factor-α Primary sclerosing cholangitis Ulcerative colitis C-Maf inducing protein Glutamate decarboxylase 1 Nucleoside diphosphate kinase 1 CDP-diacylglycerol synthase 1 Cyclin-dependent kinase regulatory subunit 1 Ubiquitin-conjugating enzyme E2C Serine protease inhibitor B1 Metastasis associated lung adenocarcinoma transcript 1 Teratocarcinoma-derived growth factor 1 (TDGF-1) Perihilar cholangiocarcinoma Aminopeptidase N Pantetheinase Polymeric immunoglobulin receptor Fibrinogen gamma chain Alpha1-acid glycoprotein 1 S100 calcium binding protein A8 Intrahepatic cholangiocarcinoma Perihilar cholangiocarcinoma Distal cholangiocarcinoma Isocitrate dehydrogenase1/2 Kirsten ratsarcoma viral oncogene homolog BRCA1 associated protein 1 Tumor protein p53 Fibroblast growth factor receptor Protein kinase cAMP-activated catalytic subunit alpha Protein kinase cAMP-activated catalytic subunit beta E74 like ETS transcription factor 3 Adipose tissue-derived mesenchymal stem cells Mechanistic target of rapamycin kinase Banales JM, Cardinale V, Carpino G, Marzioni M, Andersen JB, Invernizzi P, et al. Expert consensus document: Cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol. 2016;13(5):261–80. Banales JM, Marin JJG, Lamarca A, Rodrigues PM, Khan SA, Roberts LR, et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557–88. Cambridge WA, Fairfield C, Powell JJ, Harrison EM, Soreide K, Wigmore SJ, et al. Meta-analysis and meta-regression of survival after liver transplantation for unresectable perihilar cholangiocarcinoma. Ann Surg. 2021;273(2):240–50. Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71(1):104–14. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478). Guay C, Regazzi R. Exosomes as new players in metabolic organ cross-talk. Diabetes Obes Metab. 2017;19(Suppl 1):137–46. Budnik V, Ruiz-Canada C, Wendler F. Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci. 2016;17(3):160–72. Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14(3):195–208. Liu J, Ren L, Li S, Li W, Zheng X, Yang Y, et al. The biology, function, and applications of exosomes in cancer. Acta Pharm Sin B. 2021;11(9):2783–97. Bai M, Fu W, Su G, Cao J, Gao L, Huang C, et al. The role of extracellular vesicles in cholangiocarcinoma. Cancer Cell Int. 2020;20(1):435. van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19(4):213–28. Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18(1):75. Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21(1):9–17. Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal. 2021;19(1):47. Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3. Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in Exosome Isolation Techniques. Theranostics. 2017;7(3):789–804. Zhu L, Sun HT, Wang S, Huang SL, Zheng Y, Wang CQ, et al. Isolation and characterization of exosomes for cancer research. J Hematol Oncol. 2020;13(1):152. Witwer KW, Soekmadji C, Hill AF, Wauben MH, Buzas EI, Di Vizio D, et al. Updating the MISEV minimal requirements for extracellular vesicle studies: building bridges to reproducibility. J Extracell Vesicles. 2017;6(1):1396823. Lotvall J, Hill AF, Hochberg F, Buzas EI, Di Vizio D, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3:26913. Szatanek R, Baj-Krzyworzeka M, Zimoch J, Lekka M, Siedlar M, Baran J. The Methods of Choice for Extracellular Vesicles (EVs) Characterization. Int J Mol Sci. 2017;18(6). Saveyn H, De Baets B, Thas O, Hole P, Smith J, Van der Meeren P. Accurate particle size distribution determination by nanoparticle tracking analysis based on 2-D Brownian dynamics simulation. J Colloid Interface Sci. 2010;352(2):593–600. Chiriaco MS, Bianco M, Nigro A, Primiceri E, Ferrara F, Romano A, et al. Lab-on-Chip for Exosomes and Microvesicles Detection and Characterization. Sensors (Basel). 2018;18(10). Mathieu M, Nevo N, Jouve M, Valenzuela JI, Maurin M, Verweij FJ, et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat Commun. 2021;12(1):4389. Chen H, Chengalvala V, Hu H, Sun D. Tumor-derived exosomes: Nanovesicles made by cancer cells to promote cancer metastasis. Acta Pharm Sin B. 2021;11(8):2136–49. Zhou B, Xu K, Zheng X, Chen T, Wang J, Song Y, et al. Application of exosomes as liquid biopsy in clinical diagnosis. Signal Transduct Target Ther. 2020;5(1):144. Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5(1):145. Li L, Zhao J, Zhang Q, Tao Y, Shen C, Li R, et al. Cancer Cell-Derived Exosomes Promote HCC Tumorigenesis Through Hedgehog Pathway. Front Oncol. 2021;11:756205. Zhou J, Wang H, Sun Q, Liu X, Wu Z, Wang X, et al. miR-224-5p-enriched exosomes promote tumorigenesis by directly targeting androgen receptor in non-small cell lung cancer. Mol Ther Nucleic Acids. 2021;23:1217–28. Xue P, Huang S, Han X, Zhang C, Yang L, **ao W, et al. Exosomal miR-101–3p and miR-423–5p inhibit medulloblastoma tumorigenesis through targeting FOXP4 and EZH2. Cell Death Differ. 2021. He L, Zhu W, Chen Q, Yuan Y, Wang Y, Wang J, et al. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics. 2019;9(26):8206–20. Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, et al. Cancer-derived exosomal miR-25-3p promotes pre-metastatic niche formation by inducing vascular permeability and angiogenesis. Nat Commun. 2018;9(1):5395. Tang MKS, Yue PYK, Ip PP, Huang RL, Lai HC, Cheung ANY, et al. Soluble E-cadherin promotes tumor angiogenesis and localizes to exosome surface. Nat Commun. 2018;9(1):2270. Fang T, Lv H, Lv G, Li T, Wang C, Han Q, et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun. 2018;9(1):191. Chen X, Zhou J, Li X, Wang X, Lin Y, Wang X. Exosomes derived from hypoxic epithelial ovarian cancer cells deliver microRNAs to macrophages and elicit a tumor-promoted phenotype. Cancer Lett. 2018;435:80–91. Li YY, Tao YW, Gao S, Li P, Zheng JM, Zhang SE, et al. Cancer-associated fibroblasts contribute to oral cancer cells proliferation and metastasis via exosome-mediated paracrine miR-34a-5p. EBioMedicine. 2018;36:209–20. Wu J, Gao W, Tang Q, Yu Y, You W, Wu Z, et al. M2 Macrophage-Derived Exosomes Facilitate HCC Metastasis by Transferring alphaM beta2 Integrin to Tumor Cells. Hepatology. 2021;73(4):1365–80. Park JE, Dutta B, Tse SW, Gupta N, Tan CF, Low JK, et al. Hypoxia-induced tumor exosomes promote M2-like macrophage polarization of infiltrating myeloid cells and microRNA-mediated metabolic shift. Oncogene. 2019;38(26):5158–73. Zhang PF, Gao C, Huang XY, Lu JC, Guo XJ, Shi GM, et al. Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma. Mol Cancer. 2020;19(1):110. Lu Z, Zuo B, **g R, Gao X, Rao Q, Liu Z, et al. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J Hepatol. 2017;67(4):739–48. **e F, Xu M, Lu J, Mao L, Wang S. The role of exosomal PD-L1 in tumor progression and immunotherapy. Mol Cancer. 2019;18(1):146. Sun C, Mezzadra R, Schumacher TN. Regulation and Function of the PD-L1 Checkpoint. Immunity. 2018;48(3):434–52. Yin Z, Yu M, Ma T, Zhang C, Huang S, Karimzadeh MR, et al. Mechanisms underlying low-clinical responses to PD-1/PD-L1 blocking antibodies in immunotherapy of cancer: a key role of exosomal PD-L1. J Immunother Cancer. 2021;9(1). Zhang K, Chen J, Li C, Yuan Y, Fang S, Liu W, et al. Exosome-mediated transfer of SNHG7 enhances docetaxel resistance in lung adenocarcinoma. Cancer Lett. 2021. Zheng P, Chen L, Yuan X, Luo Q, Liu Y, **e G, et al. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017;36(1):53. **n L, Zhou LQ, Liu C, Zeng F, Yuan YW, Zhou Q, et al. Transfer of LncRNA CRNDE in TAM-derived exosomes is linked with cisplatin resistance in gastric cancer. EMBO Rep. 2021:e52124. Binenbaum Y, Fridman E, Yaari Z, Milman N, Schroeder A, Ben David G, et al. Transfer of miRNA in Macrophage-Derived Exosomes Induces Drug Resistance in Pancreatic Adenocarcinoma. Cancer Res. 2018;78(18):5287–99. Zhu X, Shen H, Yin X, Yang M, Wei H, Chen Q, et al. Macrophages derived exosomes deliver miR-223 to epithelial ovarian cancer cells to elicit a chemoresistant phenotype. J Exp Clin Cancer Res. 2019;38(1):81. Zhang H, Deng T, Liu R, Ning T, Yang H, Liu D, et al. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol Cancer. 2020;19(1):43. Han M, Hu J, Lu P, Cao H, Yu C, Li X, et al. Exosome-transmitted miR-567 reverses trastuzumab resistance by inhibiting ATG5 in breast cancer. Cell Death Dis. 2020;11(1):43. Salehi M, Sharifi M. Exosomal miRNAs as novel cancer biomarkers: Challenges and opportunities. J Cell Physiol. 2018;233(9):6370–80. Li W, Li C, Zhou T, Liu X, Liu X, Li X, et al. Role of exosomal proteins in cancer diagnosis. Mol Cancer. 2017;16(1):145. Zhou CY, Dong YP, Sun X, Sui X, Zhu H, Zhao YQ, et al. High levels of serum glypican-1 indicate poor prognosis in pancreatic ductal adenocarcinoma. Cancer Med. 2018;7(11):5525–33. Conde-Vancells J, Rodriguez-Suarez E, Gonzalez E, Berisa A, Gil D, Embade N, et al. Candidate biomarkers in exosome-like vesicles purified from rat and mouse urine samples. Proteomics Clin Appl. 2010;4(4):416–25. Kitdumrongthum S, Metheetrairut C, Charoensawan V, Ounjai P, Janpipatkul K, Panvongsa W, et al. Dysregulated microRNA expression profiles in cholangiocarcinoma cell-derived exosomes. Life Sci. 2018;210:65–75. Haga H, Yan IK, Takahashi K, Wood J, Zubair A, Patel T. Tumour cell-derived extracellular vesicles interact with mesenchymal stem cells to modulate the microenvironment and enhance cholangiocarcinoma growth. J Extracell Vesicles. 2015;4:24900. Qin X, Lu M, Li G, Zhou Y, Liu Z. Downregulation of tumor-derived exosomal miR-34c induces cancer-associated fibroblast activation to promote cholangiocarcinoma progress. Cancer Cell Int. 2021;21(1):373. Li L, Piontek K, Ishida M, Fausther M, Dranoff JA, Fu R, et al. Extracellular vesicles carry microRNA-195 to intrahepatic cholangiocarcinoma and improve survival in a rat model. Hepatology. 2017;65(2):501–14. Ota Y, Takahashi K, Otake S, Tamaki Y, Okada M, Aso K, et al. Extracellular vesicle-encapsulated miR-30e suppresses cholangiocarcinoma cell invasion and migration via inhibiting epithelial-mesenchymal transition. Oncotarget. 2018;9(23):16400–17. Wang S, Hu Y, Lv X, Li B, Gu D, Li Y, et al. Circ-0000284 arouses malignant phenotype of cholangiocarcinoma cells and regulates the biological functions of peripheral cells through cellular communication. Clin Sci (Lond). 2019;133(18):1935–53. Xu Y, Leng K, Yao Y, Kang P, Liao G, Han Y, et al. A Circular RNA, Cholangiocarcinoma-Associated Circular RNA 1, Contributes to Cholangiocarcinoma Progression, Induces Angiogenesis, and Disrupts Vascular Endothelial Barriers. Hepatology. 2021;73(4):1419–35. Scavo MP, Depalo N, Rizzi F, Ingrosso C, Fanizza E, Chieti A, et al. FZD10 Carried by Exosomes Sustains Cancer Cell Proliferation. Cells. 2019;8(8). Dutta S, Reamtong O, Panvongsa W, Kitdumrongthum S, Janpipatkul K, Sangvanich P, et al. Proteomics profiling of cholangiocarcinoma exosomes: A potential role of oncogenic protein transferring in cancer progression. Biochim Biophys Acta. 2015;1852(9):1989–99. Chen JH, **ang JY, Ding GP, Cao LP. Cholangiocarcinoma-derived exosomes inhibit the antitumor activity of cytokine-induced killer cells by down-regulating the secretion of tumor necrosis factor-alpha and perforin. J Zhejiang Univ Sci B. 2016;17(7):537–44. Lapitz A, Arbelaiz A, O'Rourke CJ, Lavin JL, Casta A, Ibarra C, et al. Patients with Cholangiocarcinoma Present Specific RNA Profiles in Serum and Urine Extracellular Vesicles Mirroring the Tumor Expression: Novel Liquid Biopsy Biomarkers for Disease Diagnosis. Cells. 2020;9(3). Shen L, Chen G, **a Q, Shao S, Fang H. Exosomal miR-200 family as serum biomarkers for early detection and prognostic prediction of cholangiocarcinoma. Int J Clin Exp Pathol. 2019;12(10):3870–6. Hu C, Zhang Y, Zhang M, Li T, Zheng X, Guo Q, et al. Exosomal Cripto-1 Serves as a Potential Biomarker for Perihilar Cholangiocarcinoma. Front Oncol. 2021;11:730615. Ge X, Wang Y, Nie J, Li Q, Tang L, Deng X, et al. The diagnostic/prognostic potential and molecular functions of long non-coding RNAs in the exosomes derived from the bile of human cholangiocarcinoma. Oncotarget. 2017;8(41):69995–70005. Xue XY, Liu YX, Wang C, Gu XJ, Xue ZQ, Zang XL, et al. Identification of exosomal miRNAs as diagnostic biomarkers for cholangiocarcinoma and gallbladder carcinoma. Signal Transduct Target Ther. 2020;5(1):77. Weeraphan C, Phongdara A, Chaiyawat P, Diskul-Na-Ayudthaya P, Chokchaichamnankit D, Verathamjamras C, et al. Phosphoproteome Profiling of Isogenic Cancer Cell-Derived Exosome Reveals HSP90 as a Potential Marker for Human Cholangiocarcinoma. Proteomics. 2019;19(12):e1800159. Ikeda C, Haga H, Makino N, Inuzuka T, Kurimoto A, Ueda T, et al. Utility of Claudin-3 in extracellular vesicles from human bile as biomarkers of cholangiocarcinoma. Sci Rep. 2021;11(1):1195. Arbelaiz A, Azkargorta M, Krawczyk M, Santos-Laso A, Lapitz A, Perugorria MJ, et al. Serum extracellular vesicles contain protein biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Hepatology. 2017;66(4):1125–43. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–81. Shroff RT, Javle MM, **ao L, Kaseb AO, Varadhachary GR, Wolff RA, et al. Gemcitabine, Cisplatin, and nab-Paclitaxel for the Treatment of Advanced Biliary Tract Cancers: A Phase 2 Clinical Trial. JAMA Oncol. 2019;5(6):824–30. Wang J, Ilyas S. Targeting the tumor microenvironment in cholangiocarcinoma: implications for therapy. Expert Opin Investig Drugs. 2021;30(4):429–38. Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15(2):95–111. Farshidfar F, Zheng S, Gingras MC, Newton Y, Shih J, Robertson AG, et al. Integrative Genomic Analysis of Cholangiocarcinoma Identifies Distinct IDH-Mutant Molecular Profiles. Cell Rep. 2017;18(11):2780–94. Nakamura H, Arai Y, Totoki Y, Shirota T, Elzawahry A, Kato M, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015;47(9):1003–10. Tabernero J, Bahleda R, Dienstmann R, Infante JR, Mita A, Italiano A, et al. Phase I Dose-Escalation Study of JNJ-42756493, an Oral Pan-Fibroblast Growth Factor Receptor Inhibitor, in Patients With Advanced Solid Tumors. J Clin Oncol. 2015;33(30):3401–8. Javle M, Roychowdhury S, Kelley RK, Sadeghi S, Macarulla T, Weiss KH, et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol Hepatol. 2021;6(10):803–15. Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer. 2015;112(9):1421–7. Piha-Paul SA, Oh DY, Ueno M, Malka D, Chung HC, Nagrial A, et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int J Cancer. 2020;147(8):2190–8. Gilligan KE, Dwyer RM. Engineering Exosomes for Cancer Therapy. Int J Mol Sci. 2017;18(6). Bandiera S, Pfeffer S, Baumert TF, Zeisel MB. miR-122–a key factor and therapeutic target in liver disease. J Hepatol. 2015;62(2):448–57. Lou G, Song X, Yang F, Wu S, Wang J, Chen Z, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8:122. Lou G, Chen L, **a C, Wang W, Qi J, Li A, et al. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J Exp Clin Cancer Res. 2020;39(1):4. Zhou Y, Zhou W, Chen X, Wang Q, Li C, Chen Q, et al. Bone marrow mesenchymal stem cells-derived exosomes for penetrating and targeted chemotherapy of pancreatic cancer. Acta Pharm Sin B. 2020;10(8):1563–75. Chaiyadet S, Sotillo J, Krueajampa W, Thongsen S, Brindley PJ, Sripa B, et al. Vaccination of hamsters with Opisthorchis viverrini extracellular vesicles and vesicle-derived recombinant tetraspanins induces antibodies that block vesicle uptake by cholangiocytes and reduce parasite burden after challenge infection. PLoS Negl Trop Dis. 2019;13(5):e0007450. Gao Y, Zhang H, Zhou N, Xu P, Wang J, Gao Y, et al. Methotrexate-loaded tumour-cell-derived microvesicles can relieve biliary obstruction in patients with extrahepatic cholangiocarcinoma. Nat Biomed Eng. 2020;4(7):743–53. Luo C, **n H, Zhou Z, Hu Z, Sun R, Yao N, et al. Tumor-Derived Exosomes Induce Immunosuppressive Macrophages to Foster Intrahepatic Cholangiocarcinoma Progression. Hepatology. 2022. Chen S, Chen Z, Li Z, Li S, Wen Z, Cao L, et al. Tumor-associated macrophages promote cholangiocarcinoma progression via exosomal Circ_0020256. Cell Death Dis. 2022;13(1):94. Thanks for the support from National Natural Science Foundation of China (No.81874062, 82072730), and Youth Program of National Natural Science Foundation of China (81902439). And thanks for two correspondence authors' wholehearted guidance and other author's cooperation. The work was supported by National Natural Science Foundation of China (No. 81874062, 82072730), and Youth Program of National Natural Science Foundation of China (81902439). KZ and WY designed the review. KZ drafted the manuscript. WY and J-MW participated in the critical revision of the manuscript. X-YL, Y-XS, YL, PQ, Z-DD participated in the search for the articles cited. All authors read and approved the final manuscript. Not applicable. Not applicable. The authors declare no conflict of interest. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data. Zhao, K., Li, X., Shi, Y. et al. Exosomes in the tumor microenvironment of cholangiocarcinoma: current status and future perspectives.
J Transl Med 20, 117 (2022). https://doi.org/10.1186/s12967-022-03294-x Received: Accepted: Published: DOI: https://doi.org/10.1186/s12967-022-03294-xAvailability of data and materials
Abbreviations
References
Acknowledgements
Funding
Author information
Authors and Affiliations
Contributions
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Consent for publication
Competing interests
Additional information
Publisher's Note
Rights and permissions
About this article
Cite this article
Keywords