Abstract
C-type lectin-like receptor-2 (CLEC-2) is a member of the C-type lectin superfamily of cell surface receptors. The first confirmed endogenous and exogenous ligands of CLEC-2 are podoplanin and rhodocytin, respectively. CLEC-2 is expressed on the surface of platelets, which participates in platelet activation and aggregation by binding with its ligands. CLEC-2 and its ligands are involved in pathophysiological processes, such as atherosclerosis, cancer, inflammatory thrombus status, maintenance of vascular wall integrity, and cancer-related thrombosis. In the last 5 years, different anti- podoplanin antibody types have been developed for the treatment of cancers, such as glioblastoma and lung cancer. New tests and new diagnostics targeting CLEC-2 are also discussed. CLEC-2 mediates thrombosis in various pathological states, but CLEC-2-specific deletion does not affect normal hemostasis, which would provide a new therapeutic tool for many thromboembolic diseases. The CLEC-2-podoplanin interaction is a target for cancer treatment. CLEC-2 may be applied in clinical practice and play a therapeutic role.
Similar content being viewed by others
Background
C-type lectin-like receptor 2 (CLEC-2) was first reported in 2000. CLEC-2 is a 32 kDa type II transmembrane protein that belongs to the C-type lectin superfamily receptor [1]. However, its expression in platelets was unknown until 2006 when Katsue Suzui-Inoue et al. described CLEC-2 as the first C-type lectin receptor found on platelets [2].
The most important ligands of CLEC-2 include two exogenous ligands rhodocytin [2] and the human immunodeficiency virus (HIV) [3] as well as an endogenous ligand, podoplanin (PDPN) [4].
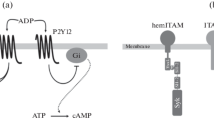
The arachidonic acid (AA), adenosine diphosphate (ADP), and platelet-activating factor pathways are currently considered as the three classical pathways of platelet activation and aggregation. As for CLEC-2, it signals through phosphorylation of a single cytoplasmic YXXL sequence known as a hem-immunoreceptor tyrosine-based activation motif (hemITAM). CLEC-2 is dependent on the activation of spleen tyrosine kinase (Syk), leading to phosphorylation of downstream adapter proteins such as SH2 domain-containing leukocyte protein of 76 kDa (SLP-76) and phospholipase Cγ2 (PLCγ2), which increases calcium concentration and activates platelets [2, 5,6,7]. In humans, this process depends highly on the positive feedback exerted by platelet-derived ADP and thromboxane A2(TXA2) [8]. TXA2 enhances the phosphorylation of Syk and PLCγ2 [9]. Furthermore, CLEC-2 does not entirely depend on hemITAM signaling in hemostasis and thrombosis [10]. T-cell ubiquitin ligand-2 (TULA-2), a protein tyrosine phosphatase, negatively regulates CLEC-2 signaling. TULA-2 deficiency leads to the enhancement of the downstream Syk phosphorylation of CLEC-2 [11].
CLEC-2 has many other physiological and pathological functions in addition to participating in platelet activation. For instance, PDPN promotes tumor metastasis and progression by activating platelets after binding to CLEC-2 [12]. Based on the relationship between CLEC-2 and diseases, blocking the interaction between CLEC-2 and its ligands may be a new target for treating these diseases. Researchers have recently developed many anti-PDPN antibodies and extracted traditional Chinese medicine components to treat tumors and thrombosis. Their effects have been proven in animal experiments.
This review introduces the coding gene, expression in various cells, structure, ligand, physiological and pathological effects, and new drug research progress of CLEC-2. We mainly focus on new drug development.
Brief introduction of CLEC-2
Coding gene, expression, and structure
The gene encoding CLEC-2 is located on chromosome 12 within approximately 100 kb [1, 13]. CLEC-2 was first cloned from human bone marrow cells, and its cDNA was first reported to be selectively expressed on hepatocytes and some bone marrow-derived blood cells, such as monocytes, dendritic cells, and granulocytes [1]. Later, CLEC-2 was found to be highly expressed in platelets [2] and megakaryocytes [14]. A previous study has demonstrated that approximately 20% of phenotypical long-term hematopoietic stem cells (LT-HSCs) expressed CLEC-2. CLEC-2high LT-HSCs were mainly involved in early megakaryopoiesis and served as a reserve for emergency thrombopoiesis under stress conditions [15]. However, CLEC-2 expression in human neutrophils has not been determined, and further studies are warranted to determine whether CLEC-2 exerts a regulatory effect on human polymorphonuclear leukocytes or other leukocytes [16].
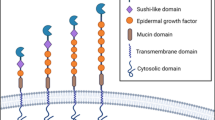
As for the structure of CLEC-2, it is a 229-amino acid type II transmembrane receptor that is composed of an extracellular ligand-binding C-type lectin-like domain, stalk region, single transmembrane helix, and short cytoplasmic tail [17–18]. The tail contains a YXXL sequence, two conserved serine sequences at positions 21 and 27, and a partially conserved threonine sequence at position 9. The YXXL sequence is critical for CLEC-2 signaling [19]. CLEC-2 regulates Syk by forming dimers on the platelet surface. The two tandem SH2 domains possessed by Syk each bind to a single phosphorylated YXXL sequence on the cytoplasmic tail of CLEC-2. That is, CLEC-2 binds Syk in a 2:1 manner. CLEC-2 acts as a dimer to mediate platelet activation and aggregation [20].
CLEC-2 and its ligands
Exogenous ligands
There are several types of exogenous ligands binding to CLEC-2. The first is the C-type lectin snake venom toxin, rhodocytin (also called aggretin), identified via chromatographic analysis [2]. Rhodocytin, a heterodimeric C-type lectin, binds to CLEC-2 to activate platelet signaling pathways [21]. Subsequently, diesel particles and sulfated polysaccharides, dextran sulfate and fucoidan, were discovered as exogenous ligands of CLEC-2 [21–22].
Katacine was a novel small-molecule exogenous ligand for CLEC-2 with a certain degree of specificity. It is a nonpolymeric proanthocyanidin extracted from the Polygonaceae family of flowering plants known as the knotweed family, which depends on Syk and Src to activate human platelets. This finding contributes to a better understanding of the role of CLEC-2 in human platelet activation and provides new ideas for further development of potential compounds with agonist effects on CLEC-2 [23]. Another recently identified exogenous ligand was galectin-9 (Gal-9). Gal-9, a family of carbohydrate-binding proteins with a broad range of immunomodulatory actions, has been reported to activate platelets. Gal-9 stimulated tyrosine phosphorylation of CLEC-2 and downstream proteins, inducing platelet activation [24].
Endogenous ligand
PDPN is the significant endogenous ligand of CLEC-2. PDPN is a type I transmembrane glycoprotein [25]. The extracellular domain of PDPN contains many serine and threonine residues as potential O-glycosylation sites. The binding of PDPN to CLEC-2 depends on the glycosylation of residues at the O-glycosylation site of PDPN, which plays a crucial role in platelet aggregation and tumor metastasis [4, 26]. In addition, PDPN binding to CLEC-2 is involved in venous thrombosis, inflammation in atherosclerosis, and wound repair. Furthermore, the interaction of CLEC-2 and PDPN promotes blood/lymphatic separation during embryonic development, maintains lymph node vascular integrity, and optimates adaptive immune response [27]. Other recently identified endogenous ligands are the smooth muscle calcium-binding protein S100A13 [28], hemin [29], and human Dectin-1 [30].
The physiological and pathological effects of CLEC-2
Physiological effects
CLEC-2 plays a central role in the formation of cerebrovascular [31] and blood/lymphatic vessel separation [32] during embryonic development. It interacts with PDPN expressed on neuroepithelial cells to induce platelet adhesion, aggregation, and secretion, which ultimately mediate the maturation and integrity of cerebrovascular and prevent hemorrhage [31]. CLEC-2 is involved in the maintenance of vascular integrity in inflammatory states. However, the mechanism of CLEC-2 activation is currently uncertain [33].
Current studies have demonstrated that CLEC-2 plays a vital role in liver regeneration. CLEC-2-associated liver regeneration was thought to be caused by the interaction between liver sinusoidal endothelial cells and platelets. CLEC-2 interacts with its endogenous ligand PDPN to induce IL-6 production, which ultimately promotes liver regeneration [34].
Role of CLEC-2 in diseases
Some ligands have been demonstrated to interact with CLEC-2 to promote thrombus formation when the plaque is not ruptured. S100 calcium-binding protein A13 (S100A13) is expressed in the luminal surface of smooth muscle cells at the early stage of atherosclerosis or under certain pathological conditions. It activates platelets through CLEC-2 to promote thrombosis. CLEC-2 also binds to smooth muscle cells in normal arterial walls. However, normal arterial walls do not express PDPN or S100A13. There may be other, as yet undiscovered CLEC-2 ligands on normal vessel walls [28]. After plaque rupture in patients with advanced atherosclerosis, PDPN expressed in the plaque binds to CLEC-2 to induce platelet activation, thereby accelerating arterial thrombosis formation [35].
Binding of PDPN to CLEC-2 plays an important role in tumor growth and metastasis. PDPN is expressed in various cancer cells, such as ovarian cancer, hematologic tumors, glioblastoma, and osteosarcoma [36,37,38,39]. It also facilitates tumor metastasis by promoting an immunosuppressive microenvironment [40]. CLEC-2 bound to CpG oligodeoxynucleotides (CpG ODNs) resulted in platelet activation. CpG ODNs are short, single-stranded DNA molecules resembling bacterial DNA. CLEC-2 may be a target for adverse events in at-risk cancer patients treated with CpG ODNs [41]. Interestingly, the expression of CLEC-2 downregulated in gastric cancer [42] and liver cancer cells [43]. CLEC-2 exerts a protective effect on both cancers, suppressing cancer invasiveness and expressing epithelial-mesenchymal transition. In conclusion, CLEC-2 plays different roles in different cancers.
CLEC-2 plays a crucial role in vascular inflammation. The interaction of PDPN and CLEC-2 not only contributes to the formation of sepsis-related immune thrombosis [44] but also restricts further deterioration of sepsis by controlling cytokine storm [45], regulating immune cell infiltration [46] and inducing the release of complement inhibitors [48]. Deep vein thrombosis (DVT) is a thromboinflammatory disorder develo** largely as sterile inflammation in the vessel wall. Payne et al. found that CLEC-2 interacted with PDPN, which was upregulated in the vein wall, to promote thrombosis [49].
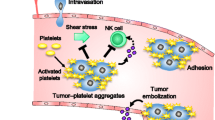
CLEC-2 is also involved in tumor-associated thrombosis. Shirai et al. found CLEC-2 interacted with PDPN to promote thrombus formation in tumor blood vessels and indirectly induced tumor cell proliferation in melanoma [36]. High PDPN expression in cancer cells induced platelet aggregation and was associated with an increased risk of venous thromboembolism (VTE) and poor prognosis [50–51].
The binding of PDPN to CLEC-2 was found to inhibit T cells by using a mouse model of experimental autoimmune encephalomyelitis. PDPN on T cells was overexpressed on Th17 cells to control inflammation. PDPN was a marker of nonpathogenic Th17 [52–53]. In rheumatoid arthritis, PDPN was overexpressed in Th17 cells and fibroblasts, which significantly increased IL-17 secretion and induced inflammation [54]. The results indicated that PDPN was a marker of pathogenic Th17, mainly depending on the type of inflammation.
The relationship between CLEC-2 and major diseases is summarized in Fig. 1.
CLEC-2 expressed on the platelet surface interacts with PDPN expressed on different cell surfaces to mediate diseases such as tumor progression and metastasis, tumor-associated thrombosis, venous thrombosis, arterial thrombosis, and immune diseases. Furthermore, sCLEC-2 levels are elevated in patients with COVID-19 and coronary artery disease.
Created with MedPeer (www.medpeer.cn).
CLEC-2, as a target in drug research
New drugs
In this subsection, we will mainly discuss research on new drugs. However, new drugs are still being developed in preclinical studies. We expect to enter the clinical stage for the following new compounds or older drugs with new indications in the future. The preclinical studies are presented in Table 1 at the end of the article.
Tumor metastasis and thrombosis
Anti-PDPN and Anti-CLEC-2 antibodies
As for anti-PDPN antibodies, there can be divided into human–mouse chimeric antibodies, monoclonal antibodies, and neutralizing antibodies. The human–mouse chimeric antibodies, NZ-12 [55], chLpMab-23 [56], and chLpMab-2 [57] have been developed. All these antibodies showed high response to cancer. For example, NZ-12, the constant regions of which consist of IgG1 heavy chain and lambda light chain. NZ-12 significantly increased antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) activities against glioblastoma and lung cancer. NZ-12 may be used for the treatment of PDPN-expressing brain cancer and lung cancer [55]. In terms of monoclonal antibodies, researchers developed LpMab-21 [58], LpMab-23 [59], P1027, NZ-12f [60], P2-0, MS-1, MS-3, and MS-4 [61]. For instance, one patent discovers a novel domain of PDPN, the PLAG4 domain, which is important for PDPN binding to CLEC-2. The anti-PDPN monoclonal antibodies recognizing this region are P2-0, MS-1, MS-3, and MS-4. These antibodies suppressed the growth of the tumor [61]. Takemoto et al. developed neutralizing antibodies PG4D2 and AP201 against the interaction of PDPN and CLEC-2, both of which exerted inhibitory effects on the growth and metastasis of osteosarcoma. These antibodies are a new therapeutic strategy for PDPN-positive osteosarcoma [62].
Moreover, some anti-PDPN monoclonal antibodies identified patients with PDPN-positive tumors. A novel anti-PDPN monoclonal antibody, LpMab-7, had high sensitivity to detect metastatic lesions of osteosarcomas. It may be helpful for molecular targeting therapy for osteosarcomas [63].
Many studies on anti-PDPN antibodies have been conducted, but only one novel antibody targeting CLEC-2 has been reported. Anti-CLEC-2 monoclonal antibody 2A2B10 has been demonstrated to inhibit hematogenous metastasis and thrombosis of PDPN-positive mouse melanoma without a significant bleeding tendency [64].
Overall, existing studies have demonstrated that anti-PDPN antibodies can be developed for clinical use. In the future, finding the downstream pathways of PDPN leading to tumor metastasis will be crucial for develo** effective drugs against PDPN. Attention should be paid to potential adverse reactions when develo** new anti-PDPN antibodies as PDPN is also expressed in normal tissues. Interestingly, LpMab monoclonal antibody series have been reported to specifically recognize aberrantly glycosylated PDPN in cancer cells, thereby avoiding adverse reactions [65]. While such antibodies are promising to avoid side effects, they may not recognize PDPN upregulation in endothelial cells and monocytes during chronic inflammation. In other words, such antibodies may not prevent the adverse effects of cancer-associated thrombosis.
Small-molecule compounds
Chang et al. developed 2CP, the first platelet antagonist with CLEC-2 binding activity. 2CP has anticancer metastatic activity in vivo. It enhances the therapeutic effect of cisplatin in experimental animal models without causing bleeding risk [66].
Another small-molecule compound is cobalt hematoporphyrin (Co-HP). Tsukiji et al. obtained Co-HP through compound screening and modification, which bound to the N120 and K211 sites of CLEC-2. Co-HP at a concentration of 1.53 µM inhibited platelet aggregation mediated through CLEC-2 but not that mediated through other receptors. Co-HP binding to CLEC-2 blocked the interaction between CLEC-2 and PDPN in lung cancer. Co-HP inhibited lung cancer metastasis and arterial or venous thrombosis in vivo without increasing bleeding risk [67]. However, its low affinity, oral availability, and toxicity indicate that it is unsuitable for clinical development.
Macromolecular compounds
As mentioned above, the expression of CLEC-2 is downregulated in gastric cancer. As an exogenous ligand of CELC-2, Fucoidan is a sulfated polysaccharide extracted from the cell wall matrix of various brown seaweed, affecting many pathophysiological processes, including cancer. Fucoidan inhibited the progression of gastric cancer by upregulating the CLEC-2 level in gastric cancer cells through the transcription factor caudal type homeobox transcription factor 2, an important regulator of gut homeostasis [68]. Nonetheless, clinical trials are need to be conducted to prove its effectiveness.
Traditional Chinese medicine
As a treasure of the Chinese nation, traditional Chinese medicine has also been found to play a significant role in treating tumors by blocking the effects of CLEC-2 and PDPN. Tseng et al. showed that a polysaccharide-containing fraction (AAWAP) from the water extract of Artemisia argyi leaves irreversibly blocked the effects of PDPN and CLEC-2 in tumors in a dose-dependent manner. Notably, AAWAP is nontoxic to cells and platelets [69]. AAWAP may effectively inhibit tumor metastasis and become a potential drug for cancer treatment. Another study found that bisdemethocycurcumin and demethoxycurcumin were natural antagonists of CLEC-2 with anti-colon cancer activity through compound screening. Further studies are warranted to confirm this result in vivo and in vitro [70].
Traditional Chinese medicine has the advantages of safety, efficacy, and low toxicity. Further research on the components of Chinese medicine may also be an effective way to discover new drugs.
Atherosclerosis
Antiplatelet therapy has long been the foundation of atherosclerotic event treatment and prevention. Studies have demonstrated that Bruton’s tyrosine kinase (Btk) is located in the downstream of Syk. Btk inhibitors can be used as novel antiplatelet agents in the treatment of thrombotic diseases. The low-dose Btk inhibitor ibrutinib blocked CLEC-2-mediated platelet activation and tyrosine phosphorylation. Ibrutinib is an irreversible Btk inhibitor that increases bleeding risk [71]. This impact of ibrutinib is likely due to off-target effects on kinases such as Src family kinases, which are supported by different effects of the CLEC-2 signaling pathway at different concentrations of Ibrutinib [72]. Other specific Btk inhibitors do not have these off-target effects, which aligns with reducing bleeding side effects in patients. A recent study developed MK-1026, a reversible Btk inhibitor. It inhibited CLEC-2-mediated platelet aggregation and reduced the risk of bleeding in patients. It is also a powerful drug for treating thrombotic diseases [73].
Transient receptor potential melastatin 7 (TRPM7) kinase is a bifunctional protein that forms a constitutively active Mg2+ and Ca2+ permeable channel. The study found that TRPM7 deletion inhibited hemITAM-PLCγ2-mediated intracellular Ca2+ mobilization using a mouse model with TRPM7 kinase activity deletion. In other words, TRPM7 kinase plays a key role in CLEC-2-induced platelet activation. Furthermore, disruption of TRPM7 kinase activity did not result in intracranial hemorrhage in mice after ischemic stroke. These results suggest that TRPM7 kinase is a safety potential target for anti-arterial thrombosis [74].
Wound healing
Depletion of CLEC-2 has been proposed as a potential novel mechanism to promote skin wound healing. This phenomenon increased fibrin(ogen) deposition, reduced inflammation, and promoted angiogenesis [75]. Targeting CLEC-2 at the wound site may be a new approach to promote wound healing.
There is already a preclinical study on wound healing. Dasatinib, a pan-tyrosine kinase inhibitor, is currently used to treat chronic myeloid leukemia. Recently, Wichaiyo et al. found that dasatinib promoted skin wound healing [76]. Dasatinib inhibited tyrosine kinases Src and Syk, which are the downstream signaling molecules of CLEC-2. Therefore, dasatinib blocked CLEC-2-mediated platelet activation, leading to self-limited inflammatory bleeding and fibrinogen/fibrin deposition, associated with reduced inflammation, increased re-epithelialization, and enhanced angiogenesis.
New tests and diagnostics
Brown et al. generated a humanized CLEC-2 (hCLEC-2KI) mouse model by replacing mouse genes with human variants using CRISPR technology. In this mouse model, hCLEC-2 can be immunodepleted, addressing the limitation that the in vivo role of hCLEC-2 is not easy to study experimentally in humans. The hCLEC-2KI mouse model provides a theoretical basis for studying anti-hCLEC-2 agents in vivo [77]. Furthermore, Watanabe et al. developed a drug screening system based on the combination of a pull-down assay using a centrifugal filter unit and a slot blot assay. An immunoglobulin Fc domain-CLEC-2 fusion protein was used as a bait to capture PDPN derived from PDPN-overexpressing HeLa cells in the presence and absence of the test compound. The system effectively screened out small-molecule inhibitors for cancer and thrombosis treatment by inhibiting the PDPN–CLEC-2 interaction [78].
In recent years, an increasing number of studies have confirmed the clinical significance of CLEC-2 in cardiovascular and cerebrovascular diseases. Platelet activation releases soluble forms of platelet membrane proteins, such as soluble CLEC-2 (sCLEC-2). sCLEC-2 binds to the ligands of CLEC-2 and inhibits membrane-bound CLEC-2 activation. Fei et al. conducted a multicenter, cross-sectional study of 216 patients, including 14 cases of stable angina pectoris (SAP, non-ACS) and 202 cases of acute coronary syndrome (ACS). The results showed that the plasma levels of sCLEC-2 were significantly increased in patients with SAP (133.67 (88.76-220.09) pg/mL) and ACS (134.16 (88.88-225.81) pg/mL) compared with control groups (65.69 (55.36-143.22) pg/mL) [88].
Because CLEC-2 does not have a high-affinity ligand, the structure-based drug design is more appropriate than the ligand-based drug design. Virtual studies, HTS studies, and molecular dynamic simulations can be selected [88].
Therefore, drugs targeting CLEC-2 are expected to be antithrombotic, antiplatelet, and antimetastatic agents with minimal bleeding side effects. However, no drug targeting CLEC-2 has been found to enter clinical trials. Future studies are expected to develop platelet aggregation inhibitors with few bleeding side effects and antimetastatic drugs that inhibit cancer metastasis, as well as their candidate compounds.
Conclusion
CLEC-2 mediates thrombosis in various pathological states, but CLEC-2-specific deletion does not affect normal hemostasis, which would provide a new therapeutic tool for many thromboembolic diseases. The interaction of PDPN and CLEC-2 is a target for cancer treatment. Due to the multiple roles of CLEC-2 in regulating platelet function during disease progression, its targeting in disease must be closely monitored.
The latest drug research focuses on anti-PDPN antibodies for the treatment of various cancer types. Only one novel antibody targeting CLEC-2 has been reported. CLEC-2-binding small molecules need to be developed further. In addition, the new drug research requires clinical trials to verify its clinical significance.
Current studies had limitations. First, most current studies are animal models and clinical studies still need to be completed. Additionally, the CLEC-2 expression differs between mice and humans. In humans, CLEC-2 is only found in platelets, megakaryocytes, and the liver. In mice, CLEC-2 is also expressed in several immune cells, including dendritic cells, peripheral neutrophils, inflammatory macrophages, and B cells. Last but not least, mouse platelets express only GPVI and CLEC-2 as receptors for the ITAM pathway, whereas human platelets also contain the human immunoglobulin GFc segment II receptor (FcγRIIA) protein. Thus, the findings from mouse experiments may not be applicable to humans. In the future, further studies can be conducted from the above three aspects or through other mechanisms of action so that CLEC-2 can be more effectively applied in clinical practice and play a better therapeutic role.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- CLEC-2:
-
C-type lectin-like receptor-2
- HIV:
-
human immunodeficiency virus
- PDPN:
-
podoplanin
- AA:
-
arachidonic acid
- ADP:
-
adenosine diphosphate
- hemITAM:
-
hem-immunoreceptor tyrosine-based activation motif
- Syk:
-
spleen tyrosine kinase
- SLP-76:
-
SH2 domain-containing leukocyte protein of 76 kDa
- PLCγ2:
-
phospholipase Cγ2
- TXA2:
-
thromboxane A2
- Btk:
-
Bruton’s tyrosine kinase
- TULA-2:
-
T-cell ubiquitin ligand-2
- LT-HSCs:
-
long-term hematopoietic stem cells
- HTS:
-
high-throughput screening
- Gal-9:
-
galectin-9
- S100A13:
-
S100 calcium-binding protein A13
- CpG ODNs:
-
CpG oligodeoxynucleotides
- COVID-19:
-
coronavirus disease 2019
- vWF:
-
von Willebrand factor
- PF4:
-
platelet factor 4
- DVT:
-
deep vein thrombosis
- VTE:
-
venous thromboembolism
- ADCC:
-
antibody-dependent cellular cytotoxicity
- CDC:
-
complement-dependent cytotoxicity
- Co-HP:
-
cobalt hematoporphyrin
- AAWAP:
-
polysaccharide-containing fraction
- Btk:
-
Bruton’s tyrosine kinase
- GPVI:
-
glycoprotein VI
- TRPM7:
-
transient receptor potential melastatin 7
- hCLEC1-2KI :
-
human CLEC-2
- pCLEC-2:
-
plasma CLEC-2
- CAD:
-
coronary artery disease
- AIS:
-
acute ischemic stroke
- ACS:
-
acute coronary syndromes
- OR:
-
odds ratio
- CI:
-
confidence interval
- SARS-CoV-2:
-
severe acute respiratory syndrome coronavirus 2
- C2PAC index:
-
sCLEC-2/platelet count ratio
- SID:
-
sepsis-induced disseminated
- IDH:
-
isocitrate dehydrogenase
References
Colonna M, Samaridis J, Angman L. Molecular characterization of two novel C-type lectin-like receptors, one of which is selectively expressed in human dendritic cells. Eur J Immunol. 2000;30(2):697–704.
Suzuki-Inoue K, Fuller GL, Garcia A, Eble JA, Pohlmann S, Inoue O, et al. A novel syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107(2):542–9.
Chawaree Chaipan I, Steffen TS, Tsegaye S, Bertram I, Glowacka Y, Kato, et al. Incorporation of podoplanin into HIV released from HEK-293T cells, but not PBMC, is required for efficient binding to the attachment factor CLEC-2. Retrovirology. 2010;19(7):47.
Navarro-Nunez L, Pollitt AY, Lowe K, Latif A, Nash GB, Watson SP. Platelet adhesion to podoplanin under flow is mediated by the receptor CLEC-2 and stabilised by Src/Syk-dependent platelet signalling. Thromb Haemost. 2015;113(5):1109–20.
Severin S, Pollitt AY, Navarro-Nunez L, Nash CA, Mourao-Sa D, Eble JA, et al. Syk-dependent phosphorylation of CLEC-2: a novel mechanism of hem-immunoreceptor tyrosine-based activation motif signaling. J Biol Chem. 2011;286(6):4107–16.
Hughes CE, Pollitt AY, Mori J, Eble JA, Tomlinson MG, Hartwig JH, et al. CLEC-2 activates syk through dimerization. Blood. 2010;115(14):2947–55.
Suzuki-Inoue K, Inoue O, Ozaki Y. Novel platelet activation receptor CLEC-2: from discovery to prospects. J Thromb Haemostasis; JTH. 2011;9(Suppl 1):44–55.
Izquierdo I, Barrachina MN, Hermida-Nogueira L, Casas V, Morán LA, Lacerenza S, et al. A Comprehensive Tyrosine Phosphoproteomic Analysis reveals Novel Components of the platelet CLEC-2 Signaling Cascade. Thromb Haemost. 2020;120(2):262–76.
Badolia R, Inamdar V, Manne BK, Dangelmaier C, Eble JA, Kunapuli SP. G(q) pathway regulates proximal C-type lectin-like receptor-2 (CLEC-2) signaling in platelets. J Biol Chem. 2017;292(35):14516–31.
Haining EJ, Cherpokova D, Wolf K, Becker IC, Beck S, Eble JA, et al. CLEC-2 contributes to hemostasis independently of classical hemITAM signaling in mice. Blood. 2017;130(20):2224–8.
Kostyak JC, Mauri BR, Dangelmaier C, Patel A, Zhou Y, Eble JA, et al. TULA-2 Deficiency enhances platelet functional responses to CLEC-2 agonists. TH open: Companion J Thromb Haemostasis. 2018;2(4):e411–9.
Kato Y, Kaneko MK, Kunita A, Ito H, Kameyama A, Ogasawara S, et al. Molecular analysis of the pathophysiological binding of the platelet aggregation-inducing factor podoplanin to the C-type lectin-like receptor CLEC-2. Cancer Sci. 2008;99(1):54–61.
Sobanov Y, Bernreiter A, Derdak S, Mechtcheriakova D, Schweighofer B, Düchler M, et al. A novel cluster of lectin-like receptor genes expressed in monocytic, dendritic and endothelial cells maps close to the NK receptor genes in the human NK gene complex. Eur J Immunol. 2001;31(12):3493–503.
Finney BA, Schweighoffer E, Navarro-Nunez L, Benezech C, Barone F, Hughes CE, et al. CLEC-2 and Syk in the megakaryocytic/platelet lineage are essential for development. Blood. 2012;119(7):1747–56.
Kumode T, Tanaka H, Esipinoza JL, Rai S, Taniguchi Y, Fujiwara R, et al. C-type lectin-like receptor 2 specifies a functionally distinct subpopulation within phenotypically defined hematopoietic stem cell population that contribute to emergent megakaryopoiesis. Int J Hematol. 2022;115(3):310–21.
Kerrigan AM, Dennehy KM, Mourao-Sa D, Faro-Trindade I, Willment JA, Taylor PR, et al. CLEC-2 is a phagocytic activation receptor expressed on murine peripheral blood neutrophils. J Immunol (Baltimore Md:1950). 2009;182(7):4150–7.
Martin EM, Zuidscherwoude M, Morán LA, Di Y, García A, Watson SP. The structure of CLEC-2: mechanisms of dimerization and higher-order clustering. Platelets. 2021;32(6):733–43.
Watson AA, Brown J, Harlos K, Eble JA, Walter TS, O’Callaghan CA. The crystal structure and mutational binding analysis of the extracellular domain of the platelet-activating receptor CLEC-2. J Biol Chem. 2007;282(5):3165–72.
Séverin S, Pollitt AY, Navarro-Nuñez L, et al. Syk-dependent phosphorylation of CLEC-2: a novel mechanism of hem-immunoreceptor tyrosine-based activation motif signaling. J Biol Chem. 2011;286(6):4107–16.
Hughes CE, Sinha U, Pandey A, Eble JA, O’Callaghan CA, Watson SP. Critical role for an acidic amino acid region in platelet signaling by the HemITAM (hemi-immunoreceptor tyrosine-based activation motif) containing receptor CLEC-2 (C-type lectin receptor-2). J Biol Chem. 2013;288(7):5127–35.
Chang CH, Chung CH, Hsu CC, Huang TY, Huang TF. A novel mechanism of cytokine release in phagocytes induced by aggretin, a snake venom C-type lectin protein, through CLEC-2 ligation. J Thromb Haemostasis: JTH. 2010;8(11):2563–70.
Manne BK, Getz TM, Hughes CE, Alshehri O, Dangelmaier C, Naik UP, et al. Fucoidan is a novel platelet agonist for the C-type lectin-like receptor 2 (CLEC-2). J Biol Chem. 2013;288(11):7717–26.
Morán LA, Di Y, Sowa MA, Hermida-Nogueira L, Barrachina MN, Martin E, et al. Katacine is a new ligand of CLEC-2 that acts as a platelet agonist. Thromb Haemost. 2022;122(8):1361–8.
Zhi Z, Jooss NJ, Sun Y, Colicchia M, Slater A, Moran LA, et al. Galectin-9 activates platelet ITAM receptors glycoprotein VI and C-type lectin-like receptor-2. J Thromb Haemostasis: JTH. 2022;20(4):936–50.
Quintanilla M, Montero-Montero L, Renart J, Martin-Villar E. Podoplanin in inflammation and Cancer. Int J Mol Sci. 2019;20(3):707.
Herzog BH, Fu J, Wilson SJ, Hess PR, Sen A, McDaniel JM, et al. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature. 2013;502(7469):105–9.
Meng D, Luo M, Liu B. The role of CLEC-2 and its ligands in Thromboinflammation. Front Immunol. 2021;12:688643.
Inoue O, Hokamura K, Shirai T, Osada M, Tsukiji N, Hatakeyama K, et al. Vascular smooth muscle cells stimulate platelets and facilitate Thrombus formation through platelet CLEC-2: implications in Atherothrombosis. PLoS ONE. 2015;10(9):e0139357.
Bourne JH, Colicchia M, Di Y, Martin E, Slater A, Roumenina LT, et al. Heme induces human and mouse platelet activation through C-type-lectin-like receptor-2. Haematologica. 2021;106(2):626–9.
Haji S, Ito T, Guenther C, Nakano M, Shimizu T, Mori D et al. Human Dectin-1 is O-glycosylated and serves as a ligand for C-type lectin receptor CLEC-2. eLife. 2022;11.
Lowe KL, Finney BA, Deppermann C, Hagerling R, Gazit SL, Frampton J, et al. Podoplanin and CLEC-2 drive cerebrovascular patterning and integrity during development. Blood. 2015;125(24):3769–77.
Osada M, Inoue O, Ding G, Shirai T, Ichise H, Hirayama K, et al. Platelet activation receptor CLEC-2 regulates blood/lymphatic vessel separation by inhibiting proliferation, migration, and tube formation of lymphatic endothelial cells. J Biol Chem. 2012;287(26):22241–52.
Boulaftali Y, Hess PR, Getz TM, Cholka A, Stolla M, Mackman N, et al. Platelet ITAM signaling is critical for vascular integrity in inflammation. J Clin Investig. 2013;123(2):908–16.
Kono H, Fujii H, Suzuki-Inoue K, Inoue O, Furuya S, Hirayama K, et al. The platelet-activating receptor C-type lectin receptor-2 plays an essential role in liver regeneration after partial hepatectomy in mice. J Thromb Haemostasis: JTH. 2017;15(5):998–1008.
Asada Y, Yamashita A, Sato Y, Hatakeyama K. Pathophysiology of atherothrombosis: mechanisms of thrombus formation on disrupted atherosclerotic plaques. Pathol Int. 2020;70(6):309–22.
Shirai T, Inoue O, Tamura S, Tsukiji N, Sasaki T, Endo H, et al. C-type lectin-like receptor 2 promotes hematogenous tumor metastasis and prothrombotic state in tumor-bearing mice. J Thromb Hemostasis: JTH. 2017;15(3):513–25.
Gharahkhani R, Pourhadi M, Mirdamadi NS, Dana N, Rafiee L, Nedaeinia R, et al. Effect of Anti-podoplanin on malignant glioma cell viability, Invasion and Tumor Cell-Induced platelet aggregation. Arch Med Res. 2022;53(5):461–8.
Sasano T, Gonzalez-Delgado R, Muñoz NM, Carlos-Alcade W, Cho MS, Sheth RA, et al. Podoplanin promotes tumor growth, platelet aggregation, and venous thrombosis in murine models of ovarian cancer. J Thromb Haemostasis: JTH. 2022;20(1):104–14.
Ichikawa J, Ando T, Kawasaki T, Sasaki T, Shirai T, Tsukiji N, et al. Role of platelet C-Type lectin-like receptor 2 in promoting lung metastasis in Osteosarcoma. J bone Mineral Research: Official J Am Soc Bone Mineral Res. 2020;35(9):1738–50.
Agrawal S, Ganguly S, Hajian P, Cao JN, Agrawal A. PDGF upregulates CLEC-2 to induce T regulatory cells. Oncotarget. 2015;6(30):28621–32.
Flierl U, Nero TL, Lim B, Andrews RK, Parker MW, Gardiner EE, et al. Targeting of C-type lectin-like receptor 2 or P2Y12 for the prevention of platelet activation by immunotherapeutic CpG oligodeoxynucleotides: comment. J Thromb Haemostasis: JTH. 2018;16(1):181–5.
Wang L, Yin J, Wang X, Shao M, Duan F, Wu W, et al. C-Type Lectin-Like receptor 2 suppresses AKT Signaling and Invasive activities of Gastric Cancer cells by blocking expression of phosphoinositide 3-Kinase subunits. Gastroenterology. 2016;150(5):1183–e9516.
Critelli R, Milosa F, Faillaci F, Condello R, Turola E, Marzi L, et al. Microenvironment inflammatory infiltrate drives growth speed and outcome of hepatocellular carcinoma: a prospective clinical study. Cell Death Dis. 2017;8(8):e3017.
Hitchcock JR, Cook CN, Bobat S, Ross EA, Flores-Langarica A, Lowe KL, et al. Inflammation drives thrombosis after Salmonella infection via CLEC-2 on platelets. J Clin Investig. 2015;125(12):4429–46.
Georg F, Weber BG, Chousterman S, He AM, Fenn M, Nairz A, Anzai, et al. Interleukin-3 amplifies acute inflammation and is a potential therapeutic target in sepsis. Science. 2015;347(6227):1260–5.
Rayes J, Lax S, Wichaiyo S, Watson SK, Di Y, Lombard S, et al. The podoplanin-CLEC-2 axis inhibits inflammation in sepsis. Nat Commun. 2017;8(1):2239.
**e Z, Shao B, Hoover C, McDaniel M, Song J, Jiang M et al. Monocyte upregulation of podoplanin during early sepsis induces complement inhibitor release to protect liver function. JCI Insight. 2020;5(13).
Iba T, Wada H, Levy JH. Platelet activation and thrombosis in COVID-19. Semin Thromb Hemost. 2023;49(1):55–61.
Payne H, Ponomaryov T, Watson SP, Brill A. Mice with a deficiency in CLEC-2 are protected against deep vein thrombosis. Blood. 2017;129(14):2013–20.
Wang X, Liu B, Xu M, Jiang Y, Zhou J, Yang J, et al. Blocking podoplanin inhibits platelet activation and decreases cancer-associated venous thrombosis. Thromb Res. 2021;200:72–80.
Riedl J, Preusser M, Nazari PMS, Posch F, Panzer S, Marosi C, et al. Podoplanin expression in primary brain tumors induces platelet aggregation and increases risk of venous thromboembolism. Blood. 2017;129(13):1831–9.
Peters A, Burkett PR, Sobel RA, Buckley CD, Watson SP, Bettelli E, et al. Podoplanin negatively regulates CD4 + effector T cell responses. J Clin Investig. 2015;125(1):129–40.
Nylander AN, Ponath GD, Axisa PP, Mubarak M, Tomayko M, Kuchroo VK, et al. Podoplanin is a negative regulator of Th17 inflammation. JCI Insight. 2017;2(17):e92321.
Noack M, Ndongo-Thiam N, Miossec P. Interaction among activated lymphocytes and mesenchymal cells through podoplanin is critical for a high IL-17 secretion. Arthritis Res Ther. 2016;18:148.
Kaneko MK, Abe S, Ogasawara S, Fujii Y, Yamada S, Murata T, et al. Chimeric anti-human podoplanin antibody NZ-12 of Lambda Light Chain exerts higher antibody-dependent Cellular cytotoxicity and complement-dependent cytotoxicity compared with NZ-8 of Kappa Light Chain. Monoclon Antibodies Immunodiagnosis Immunotherapy. 2017;36(1):25–9.
Kaneko MK, Nakamura T, Kunita A, Fukayama M, Abe S, Nishioka Y, et al. ChLpMab-23: Cancer-specific human-mouse chimeric anti-podoplanin antibody exhibits Antitumor Activity via antibody-dependent Cellular cytotoxicity. Monoclon Antibodies Immunodiagnosis Immunotherapy. 2017;36(3):104–12.
Kaneko MK, Yamada S, Nakamura T, Abe S, Nishioka Y, Kunita A, et al. Antitumor activity of chLpMab-2, a human-mouse chimeric cancer-specific antihuman podoplanin antibody, via antibody-dependent cellular cytotoxicity. Cancer Med. 2017;6(4):768–77.
Kato Y, Kunita A, Fukayama M, Abe S, Nishioka Y, Uchida H, et al. Antiglycopeptide mouse monoclonal antibody LpMab-21 exerts Antitumor Activity Against Human podoplanin through antibody-dependent Cellular cytotoxicity and complement-dependent cytotoxicity. Monoclon Antibodies Immunodiagnosis Immunotherapy. 2017;36(1):20–4.
Yamada S, Ogasawara S, Kaneko MK, Kato Y. LpMab-23: a Cancer-specific monoclonal antibody against human podoplanin. Monoclon Antibodies Immunodiagnosis Immunotherapy. 2017;36(2):72–6.
Kaneko MK, Ohishi T, Nakamura T, Inoue H, Takei J, Sano M, et al. Development of Core-Fucose-Deficient Humanized and chimeric anti-human podoplanin antibodies. Monoclon Antibodies Immunodiagnosis Immunotherapy. 2020;39(5):167–74.
Naoya FUJITA. SEKIGUCHl Takaya, TAKAGl Satoshi. Anti-aggrus Monoclonal Antibody, Domain In Aggrus Which Is Required For Binding To CLEC-2, And Method For Screening For Aggrus-CLEC-2 Binding Inhibitor. EP3323831A1 (2018).
Takemoto A, Takagi S, Ukaji T, Gyobu N, Kakino M, Takami M, et al. Targeting Podoplanin for the Treatment of Osteosarcoma. Clin cancer Research: Official J Am Association Cancer Res. 2022;28(12):2633–45.
Kaneko MK, Oki H, Ogasawara S, Takagi M, Kato Y. Anti-podoplanin monoclonal antibody LpMab-7 detects metastatic lesions of Osteosarcoma. Monoclon Antibodies Immunodiagnosis Immunotherapy. 2015;34(3):154–61.
Shirai T, Inoue O, Tamura S, Tsukiji N, Sasaki T, Endo H, et al. C-type lectin-like receptor 2 promotes hematogenous tumor metastasis and prothrombotic state in tumor-bearing mice. J Thromb Haemostasis: JTH. 2017;15(3):513–25.
Takemoto A, Miyata K, Fujita N. Platelet-activating factor podoplanin: from discovery to drug development. Cancer Metastasis Rev. 2017;36(2):225–34.
Chang Y-W, Hsieh P-W, Chang Y-T, Lu M-H, Huang T-F, Chong K-Y, et al. Identification of a novel platelet antagonist that binds to CLEC-2 and suppresses podoplanin-induced platelet aggregation and cancer metastasis. Oncotarget. 2015;6(40):42733–48.
Tsukiji N, Osada M, Sasaki T, Shirai T, Satoh K, Inoue O, et al. Cobalt hematoporphyrin inhibits CLEC-2-podoplanin interaction, tumor metastasis, and arterial/venous thrombosis in mice. Blood Adv. 2018;2(17):2214–25.
Xu L, Liu F, Li C, Li S, Wu H, Guo B, et al. Fucoidan suppresses the gastric cancer cell malignant phenotype and production of TGF-β1 via CLEC-2. Glycobiology. 2020;30(5):301–11.
Tseng CP, Huang YL, Chang YW, Liao HR, Chen YL, Hsieh PW. Polysaccharide-containing fraction from Artemisia argyi inhibits tumor cell-induced platelet aggregation by blocking interaction of podoplanin with C-type lectin-like receptor 2. J food drug Anal. 2020;28(1):115–23.
Chandra Manivannan A, Bargueňo MC, Devaraju V, Sen P, Pérez-Sánchez H, Mohammed Ghilan AK et al. Curcumin-Based Inhibitors of Thrombosis and Cancer Metastasis Promoting Factor CLEC 2 from Traditional Medicinal Species Curcuma longa. Evidence-based complementary and alternative medicine: eCAM. 2022;2022:9344838.
Nicolson PLR, Nock SH, Hinds J, Garcia-Quintanilla L, Smith CW, Campos J, et al. Low-dose btk inhibitors selectively block platelet activation by CLEC-2. Haematologica. 2021;106(1):208–19.
Dangelmaier C, Vari HR, Wright M, Kostyak JC, Kunapuli SP. Clustering extent-dependent differential signaling by CLEC-2 receptors in platelets. Res Pract Thromb Haemost. 2022;6(3):e12710.
Tullemans BME, Karel MFA, Léopold V, Ten Brink MS, Baaten C, Maas SL, et al. Comparison of inhibitory effects of irreversible and reversible Btk inhibitors on platelet function. EJHaem. 2021;2(4):685–99.
Gotru SK, Chen W, Kraft P, Becker IC, Wolf K, Stritt S et al. TRPM7 kinase controls calcium responses in arterial thrombosis and stroke in mice. Arteriosclerosis, thrombosis, and vascular biology. 2018;38(2):344–52.
Wichaiyo S, Lax S, Montague SJ, Li Z, Grygielska B, Pike JA, et al. Platelet glycoprotein VI and C-type lectin-like receptor 2 deficiency accelerates wound healing by impairing vascular integrity in mice. Haematologica. 2019;104(8):1648–60.
Wichaiyo S, Svasti S, Supharattanasitthi W, Morales NP. Dasatinib induces loss of vascular integrity and promotes cutaneous wound repair in mice. J Thromb Haemostasis: JTH. 2021;19(12):3154–67.
Brown HC, Beck S, Navarro S, Di Y, Jerez EMS, Kaczmarzyk J, et al. Antibody-mediated depletion of human CLEC-2 in a novel humanized mouse model. Blood Adv. 2023;7(6):997–1000.
Watanabe N, Kidokoro M, Suzuki Y, Tanaka M, Inoue S, Tsukamoto H, et al. A pull-down and slot blot-based screening system for inhibitor compounds of the podoplanin-CLEC-2 interaction. PLoS ONE. 2019;14(9):e0222331.
Fei M, **ang L, Chai X, ** J, You T, Zhao Y, et al. Plasma soluble C-type lectin-like receptor-2 is associated with the risk of coronary artery disease. Front Med. 2020;14(1):81–90.
Wu X, Zhang W, Li H, You S, Shi J, Zhang C, et al. Plasma C-type lectin-like receptor 2 as a predictor of death and vascular events in patients with acute ischemic stroke. Eur J Neurol. 2019;26(10):1334–40.
Zhang X, Zhang W, Wu X, Li H, Zhang C, Huang Z, et al. Prognostic significance of plasma CLEC-2 (C-Type lectin-like receptor 2) in patients with Acute ischemic stroke. Stroke. 2019;50:45–52.
Wada H, Ichikawa Y, Ezaki M, Yamamoto A, Tomida M, Yoshida M, et al. Elevated plasma Soluble C-Type lectin-like receptor 2 is Associated with the worsening of Coronavirus Disease 2019. J Clin Med. 2022;11(4):985.
Hideo WADA, Masahide KAWAMURA. Method for assessing risk of hemorrhagic stroke using Soluble CLEC2. WO2022255349A1 (2022).
Ishikura H, Irie Y, Kawamura M, Hoshino K, Nakamura Y, Mizunuma M, et al. Early recognition of sepsis-induced coagulopathy using the C2PAC index: a ratio of soluble type C lectin-like receptor 2 (sCLEC-2) level and platelet count. Platelets. 2022;33(6):935–44.
Ando K, Natsumeda M, Kawamura M, Shirakawa K, Okada M, Tsukamoto Y, et al. Elevated ratio of C-type lectin-like receptor 2 level and platelet count (C2PAC) aids in the diagnosis of post-operative venous thromboembolism in IDH-wildtype gliomas. Thromb Res. 2023;223:36–43.
Suzuki-Inoue K, Kato Y, Inoue O, Kaneko MK, Mishima K, Yatomi Y, et al. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells. J Biol Chem. 2007;282(36):25993–6001.
Harbi MH, Smith CW, Nicolson PLR, Watson SP, Thomas MR. Novel antiplatelet strategies targeting GPVI, CLEC-2 and tyrosine kinases. Platelets. 2021;32(1):29–41.
Damaskinaki FN, Moran LA, Garcia A, Kellam B, Watson SP. Overcoming challenges in develo** small molecule inhibitors for GPVI and CLEC-2. Platelets. 2021;32(6):744–52.
Acknowledgements
Not applicable.
Funding
This study was funded by the National High Level Hospital Clinical Research Fund (Peking University First Hospital Science and Technology Achievement Transformation and Incubation Guidance Fund, 2023IR06), and the National Natural Science Foundation of China (82274024, 81973395, and 82073935).
Author information
Authors and Affiliations
Contributions
Q. X. developed the idea. L. S. performed a framework sorting and wrote the initial manuscript. Article was reviewed and suggested for revision by Q. X., Z. W., Z. L., G. M., Y. C. before submission. The final revisions were made by L. S.. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sun, L., Wang, Z., Liu, Z. et al. C-type lectin-like receptor 2: roles and drug target. Thrombosis J 22, 27 (2024). https://doi.org/10.1186/s12959-024-00594-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12959-024-00594-8