Abstract
Background
Nitric oxide (NO) is a ubiquitous signaling mediator in various physiological processes. However, there are less reports concerning the effects of NO on fungal secondary metabolites. Hypocrellins are effective anticancer photodynamic therapy (PDT) agents from fungal perylenequinone pigments of Shiraia. NO donor sodium nitroprusside (SNP) was used as a chemical elicitor to promote hypocrellin biosynthesis in Shiraia mycelium cultures.
Results
SNP application at 0.01–0.20 mM was found to stimulate significantly fungal production of perylenequinones including hypocrellin A (HA) and elsinochrome A (EA). SNP application could not only enhance HA content by 178.96% in mycelia, but also stimulate its efflux to the medium. After 4 days of SNP application at 0.02 mM, the highest total production (110.34 mg/L) of HA was achieved without any growth suppression. SNP released NO in mycelia and acted as a pro-oxidant, thereby up-regulating the gene expression and activity of reactive oxygen species (ROS) generating NADPH oxidase (NOX) and antioxidant enzymes, leading to the increased levels of superoxide anion (O2−) and hydrogen peroxide (H2O2). Gene ontology (GO) analysis revealed that SNP treatment could up-regulate biosynthetic genes for hypocrellins and activate the transporter protein major facilitator superfamily (MFS) for the exudation. Moreover, SNP treatment increased the proportion of total unsaturated fatty acids in the hypha membranes and enhanced membrane permeability. Our results indicated both cellular biosynthesis of HA and its secretion could contribute to HA production induced by SNP.
Conclusions
The results of this study provide a valuable strategy for large-scale hypocrellin production and can facilitate further understanding and exploration of NO signaling in the biosynthesis of the important fungal metabolites.
Similar content being viewed by others
Background
Shiraia bambusicola P. Hennings is a bambusicolous fungus parasitized on bamboo tender twigs and its fruiting bodies have been used in traditional Chinese medicine to stimulate blood circulation, relieve expectoration, cure rheumarthritis and relax muscle rigidity [1, 2]. Hypocrellins, main perylenequinone pigments isolated from the hypha and fruit-bodies of Shiraia fungi, have being developed as new non-porphyrin and reactive oxygen species (ROS)-generating photosensitizers in photodynamic therapy (PDT) for clinical application of anti-microbes, -cancers and -viruses [1, 3, 4]. Due to the difficulty of chemical synthesis of hypocrellins and the scarcity of wild fruiting bodies [5], Shiraia mycelium culture has become a promising production process of this new PDT agent [6]. More process strategies were applied to enhance hypocrellin production in the culture, including the condition optimization for the cultures [7], and application of biotic [8, 9] or abiotic elicitors [10, 11]. Nitric oxide (NO), a small molecular signal, was found to be involved in the induced hypocrellin production by a fungal elicitor from Aspergillus niger at 50 µg/mL [12] and a fungal elicitor PB90 at 5 nM [8]. The endogenous NO in S. bambusicola was also observed in extractive culture of Shiraia by Triton X-100 [13].
NO is an important regulatory molecule in mammals [14]. Recently, NO has been proved to be an essential signal in the elicitation of plant defense and secondary metabolite biosynthesis [15]. NO released by a NO donor sodium nitroprusside (SNP) could elicit plant secondary metabolites such as marjoram essential oil [16], total phenols and flavonoids in Echinacea purpurea roots [17], artemisinin in Artemisia annua hairy roots [18], and taxol in Taxus yunnanensis cells [19]. However, there are few reports on the effects of NO on fungal growth and secondary metabolism. NO may mediate L-arginine-induced conidiation of Coniothyrium minitans [20]. Wang and Higgins (2005) reported that the germination and development of Colletotrichum coccodes conidia were significantly inhibited by SNP at 100 µM [21]. A NO-releasing compound diethylenetriamine-NoNoate at 1.5 mM increased drastically the formation of cleistothecia in A. nidulans, suggesting a positive regulator of NO on fungal sexual development [22]. Zhao et al. demonstrated that NO was involved in the co-culture of Inonotus obliquus with Phellinus morii and triggered the biosynthetical pathway of phenylpropanoids, leading to an increased production of styrylpyrone derivatives [23]. SNP at 5 mM increased yield of ganoderic triterpenoid by 40.94% in submerged fermenting Ganoderma lucidum [24]. Although the endogenous NO generation was observed in S. bambusicola treated by fungal elicitors [8, 12] and Triton X-100 [13], the regulation roles of NO on fungal hypocrellin biosynthesis are still undetermined. Therefore, as a follow-up to our efforts on enhancing hypocrellin production [9, 25] and elucidating the role of NO on secondary metabolite biosynthesis [13, 26], we examined the effects of SNP on Shiraia hypocrellin biosynthesis. We also investigated the relationship between NO and elicitation responses including ROS generation, the activation of antioxidant defenses and hypocrellin production in Shiraia mycelium cultures. In this study, de novo transcriptome sequencing for Shiraia sp. S9 was performed for better understanding the NO regulation on hypocrellin biosynthesis. This study presented a novel elicitor of hypocrellin production and shed light on the relational roles of NO on the biosynthesis of secondary metabolites.
Results
SNP application released NO and enhanced hypocrellin production
After the application of SNP at 0.10 mM to 3-day-old cultures, we observed a rise of green fluorescence of NO indicator 4, 5-diaminofluorescein diacetate (DAF-2-DA) in Shiraia mycelia, whereas the relative fluorescent ration was markedly decreased by NO scavenger 2-(4-carboxyphenyl)-4, 4, 5, 5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO), indicating the release of NO in hyphae (Fig. 1a, b). On the solid medium, the concentration of fungal conidia was decreased from 17.08 × 106 to 2.43 × 106 spores/mL by SNP treatment (Fig. 2a). The pycnidium formation was suppressed by SNP (Fig. 2b). Although SNP at higher concentration (1.00 mM) inhibited the fungal growth, the diameter of fungal colony was increased on day 6 by SNP at 0.01 or 0.02 mM (Fig. 2c, d). In the liquid culture, there were not any notable changes in fungal biomass (Additional file 1: Figure S1A), pH value and sugar consumption (Additional file 1: Figure S2) in medium after SNP application at 0.10 mM. However, SNP decreased fungal pellet diameter by 16.09–25.73% (Additional file 1: Figure S1B) and the pellets were colored with dark red (Fig. 2e). The influence of SNP on hypocrellins including hypocrellin A (HA) and HC, and elsinochrome (A, B and C) in the culture was detected by HPLC (Additional file 1: Figure S3). Both the intracellular HA (1.22-fold of control) and EA (5.88-fold of control) were enhanced by SNP at 0.10 mM (Table 1). The extracellular HA was increased by 87.56%, while HC, EB and EC were not detected in cultural broth with or without SNP treatment.
NO generation in SNP-treated mycelia of Shiraia sp. S9. a Bright-field image (above) and fluorescence microscopy of DAF-2-DA-stained mycelium (below) in cultures. SNP was added at 0.10 mM on day 3 of the culture. cPTIO (0.10 mM) was added 30 min prior to SNP treatment. The photos were taken after 2 h of SNP treatment. b NO accumulation (relative intensity of fluorescent ratio) in mycelium after SNP treatment. Values are mean ± SD from three independent experiments (**p < 0.01 vs. control, ##p < 0.01 vs. SNP group)
Effects of SNP on the growth and development of Shiraia sp. S9. a Effect of SNP (0.01–1.00 mM) on the generation of conidia of S9 strain on day 8 in solid medium culture. b The morphologic characteristics of S9 strain was kept on PDA with or without SNP (0.10 mM) treatment for 8 days. c The diameter of S9 colony treated by SNP at 0.01–1.00 mM for 2–6 days. d The colony morphology of S9 strain with SNP (0.01–1.00 mM) treatment for 6 days. e Pellet morphology (15 ×) in submerged culture of S9 strain under SNP treatment at 0.10 mM on day 3. The culture was maintained in 150-mL flask containing 50 mL of the liquid medium at 150 rpm and 28℃. The red arrow indicates pycnidium. The black arrow indicates addition time. Values are mean ± SD from three independent experiments (*p < 0.05 and **p < 0.01 vs. control)
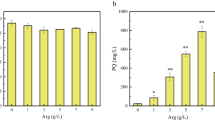
As HA is a major bioactive hypocrellin constituent in Shiraia [9], we optimized the conditions (concentration and addition time) of SNP application to obtain higher HA production. SNP at lower concentrations (0.01–0.20 mM) had no obvious impacts on fungal biomass (Additional file 1: Figure S4A), but increased HA contents in mycelium and in cultural broth. The higher HA in mycelium (9.72 mg/g DW) and in cultural broth (4.64 mg/L) were obtained at 0.02 mM and 0.20 mM, respectively (Additional file 1: Figure S4B, C). When SNP at 0.02 mM was applied on different days, the higher contents of intracellular HA (9.97 mg/g DW) and the released HA in cultural broth (4.42 mg/L) were achieved on day 3 (Additional file 1: Figure S5). Hence, SNP application at 0.02 mM on day 3 of the culture was then used for subsequent culture. The time course of the effect of SNP on HA production in 9-day-old cultures was shown in Fig. 3. Under this optimal condition, SNP application did not suppress mycelial growth (Fig. 3a), but promoted the intracellular HA contents by 73.31–178.96% (Fig. 3b) and extracellular HA accumulation by 26.09–119.26% (Fig. 3c). The total HA production was enhanced to 110.34 mg/L on day 7, a 2.65-fold increase over the control without SNP addition (Fig. 3d).
Time profiles of fungal biomass a, HA content in mycelia b, the released HA in cultural broth c and total HA production d in submerged culture of Shiraia sp. S9 under 0.02 mM SNP treatment on day 3. The culture was maintained in 150-mL flask containing 50 mL of the liquid medium at 150 rpm and 28 °C. The arrow represents the time of SNP addition. Values are mean ± SD from three independent experiments (**p < 0.01 vs. control)
SNP-induced transcriptional changes of genes for hypocrellin biosynthesis
To examine the transcriptional changes of Shiraia sp. S9 after SNP treatment, RNA-Seq experiment was subsequently performed. There were altogether 84,275 unigenes assembled with an average length of 1,011.81 bp (base pairs) and an N50 of 5,399 bp (Additional file 1: Table S1, Figure S6). 98.62%, 54.40%, 37.37%, 30.86% and 90.40% of the total unigenes were resemble to known genes reported in the current databases in Additional file 1: Table S2. A total of 571 differentially expressed genes (DEGs) were identified (Additional file 2: Table S3), including up-regulated unigenes (355, 62.17%) and down-regulated unigenes (216, 37.83%) under SNP treatment (Additional file 1: Figure S7). These DEGs were categorized into the independent classification group “biological process (BP)”, “molecular function (MF)” and “cellular component (CC)” (Additional file 2: Table S4). Compared with the annotated unigenes of BP (270 DEGs) and CC (169 DEGs) (Additional file 1: Figure S8A, B), more than 322 DEGs were categorized into MF group (Additional file 1: Figure S8C). Within the MF category, DEGs (129 unigenes) assigned to ‘catalytic activity’ (GO:0003824) were of the highest proportion and other DEGs were mainly involved in ‘oxidoreductase activity’ (GO:0016491), ‘transporter activity’ (GO:0005215) and ‘transmembrane transporter activity’ (GO:0022857) items.
Based on the reported gene clusters for hypocrellin biosynthesis [27,28,29], we explored the expression changes of DEGs associated with hypocrellin biosynthesis under SNP treatment. De novo sequencing and comparative analysis revealed 113 putative DEGs were enriched in 10 items related to fungal hypocrellin production (Additional file 2: Table S5 and S6), including ‘polyketide synthase’, ‘hydroxylase’, ‘probable metabolite transport protein’, and so forth. Among them, more than 75% DEGs which are associated with intracellular hypocrellin biosynthesis were up-regulated compared with control group (Additional file 2: Table S5 and Fig. 4a), such as laccase-like multicopper oxidase (MCO, TRINITY_DN31904_c0_g1_i4), conidial yellow pigment biosynthesis polyketide synthase (PKS, TRINITY_DN73250_c0_g1_i1), hydroxyindole-O-methyltransferase (Omef, TRINITY_DN28714_c0_g2_i1), FAD dependent oxidoreductase (FAD, TRINITY_DN33725_c0_g1_i8), phenol hydroxylase (Hyd, TRINITY_DN84020_c0_g1_i1), cytochrome P450 CYP2 subfamily (Mono, TRINITY_DN31508_c0_g2_i1), fasciclin (Fas, TRINITY_DN43991_c0_g1_i1) (Table 2). The expression levels of seven randomly selected unigenes related to hypocrellin biosynthesis were confirmed through qRT-PCR strictly (Fig. 4b), in which Hyd and PKS were more dramatically up-regulated by 59.35- and 14.59-fold, separately. The expression changes by qRT-PCR showed a similar tendency to those in transcriptome data (Table 2). In addition, it was found that more than 60% DEGs bound up with extracellular HA exportation were also up-regulated (Additional file 2: Table S6), such as major facilitator superfamily (MFS, TRINITY_DN33647_c1_g1_i8), ABC superfamily (ABC, TRINITY_DN73211_c0_g2_i1), secondary metabolites biosynthesis, transport and catabolism (MTP, TRINITY_DN30127_c0_g1_i1) (Table 2). Meanwhile, the SNP-induced up-regulation of transcriptional expression of selected unnigene MFS (TRINITY_DN33647_c1_g1_i8) from 2.40- to 7.31-fold was confirmed by qRT-PCR (Fig. 4c).
a Heat map of DEGs involved in hypocrellin biosynthesis of Shiraia sp. S9 by SNP. b Validation of the expression levels of unigenes related to hypocrellin biosynthesis of S9 strain by qRT-PCR on day 8. Hyd, TRINITY_DN84020_c0_g1_i1. PKS, TRINITY_DN73347_c0_g1_i1. MCO, TRINITY_DN31904_c0_g1_i4. FAD, TRINITY_DN33725_c0_g1_i8. Omef, TRINITY_DN28714_c0_g2_i1. Mono, TRINITY_DN33456_c1_g1_i4. Fas, TRINITY_DN43991_c0_g1_i1. c Validation of the expression levels of MFS (TRINITY_DN33647_c1_g1_i8) by qRT-PCR. The SNP treatment was the same as specified in Fig. 3. Values are mean ± SD from three independent experiments (**p < 0.01 vs. control)
As shown in Additional file 2: Table S7, a total of 6440 unigenes were classified into 55 transcription factor (TF) groups, including C2H2, Trihelix, bZIP, bHLH, MYB related and so forth. Among all the TFs identified, bHLH (12.61%), ERF (7.47%), and MYB-related TF (7.08%) were of a higher proportion. Furthermore, there were altogether 189 DEGs enriched into 27 TFs differently expressed under SNP treatment (Additional file 2: Table S8), in which 24 TFs (110 DEGs) were up-regulated while 20 TFs (53 DEGs) were down-regulated in Shiraia mycelia. Among the up-regulated TFs, ERF ranked the highest (12 DEGs), followed by C3H (12 DEGs), and MYB (11 DEGs) (Table 2). Meanwhile, in the down-regulated TFs, NAC ranked the highest (8 DEGs), followed by C2H2 (6 DEGs), and bHLH (5 DEGs). Some prominent expression changes of unigenes associated with the high proportion of TFs were listed in Table 2, including several hypothetical proteins (TRINITY_DN31904_c0_g1_i4, TRINITY_DN33640_c0_g1_i50, TRINITY_DN33640_c0_g1_i25 and TRINITY_DN28413_c0_g1_i2), two aldehyde dehydrogenases (TRINITY_DN33276_c0_g1_i17 and TRINITY_DN33276_c0_g1_i28), hexose transporter-like protein (TRINITY_DN31580_c0_g1_i1), ATP binding (TRINITY_DN31301_c0_g1_i7).
SNP-induced transcriptional changes of genes involved in membrane permeabilization
On the basis of the analysis results from GO classification (Additional file 2: Table S4), a large number of unigenes were enriched into ‘membrane’ (61 DEGs of GO:0016020), ‘transporter’ (51 DEGs of GO:0006810), ‘integral to membrane’ (36 DEGs of GO:0016021), ‘intrinsic to membrane’ (36 DEGs of GO:0031224), ‘membrane part’ (36 DEGs of GO:0,044,425), ‘transmembrane transport’ (34 DEGs of GO:0055085), ‘transporter activity’ (33 DEGs of GO:0005215) and ‘transmembrane transporter activity’ (30 DEGs of GO:0022857) items after SNP application. Hence, we explored the alteration of hyphal cell membrane permeability and membrane lipid components of Shiraia sp. S9. As shown in Fig. 5, the fluorescence was strengthened in SYTOX Green-stained cells after SNP treatment, indicating the increased permeabilization of cell membrane. Simultaneously, we found that the composition proportions of some saturated fatty acids such as palmitic (C16:0) and stearic (C18:0) were decreased by 19.08% and 23.97%, respectively (Table 3). On the contrary, the proportions of four unsaturated fatty acids including palmitoleic (C16:1), oleic (C18:1), erucic (C22:1) and nervonic (C24:1) were raised significantly, up to 6.23-fold (Table 3). Hence, the ratio of unsaturated fatty acids with saturated ones was enhanced to 0.57 in the hyphal cells of S9 strain under SNP treatment, a 4.07-fold of the control group, suggesting the enhancement of cell membrane fluidity.
Effect of SNP treatment on membrane permeability in Shiraia sp. S9 mycelia (400 ×). The 8-day-old fungal mycelium was incubated with 0.50 μM SYTOX Green for 30 min. The SNP treatment was the same as specified in Fig. 3. Panels represent bright-field images on the left and green fluorescence images on the right
SNP-induced transcriptional changes of genes involved in oxidative stress
According to the transcriptome analysis, there were 121 DEGs enriched into ‘oxidation–reduction process’ (GO:0055114), ‘oxidoreductase activity’ (GO:0016491), ‘electron carrier activity’ (GO:0009055) and ‘oxidoreductase activity, acting on paired donors, with incorporation or reduction of molecular oxygen’ (GO:0016705) items (Additional file 2: Table S4), suggesting an oxidative stress induced by SNP. By comparison between control and SNP groups (Fig. 6a), the green fluorescent signals of 2, 7-dichlorodihydroflurescein diacetate (DCFH-DA) in hyphal cells were brighter and more intense (4.72-fold, Fig. 6b), indicating the increase of ROS generation. When Shiraia sp. S9 was cultured in the presence of ROS scavenger vitamin C (Vc) and diphenyleneiodonium (DPI), an inhibitor of ROS generating enzyme NADPH oxidase (NOX) for 30 min prior to SNP application, the relative intensities of fluorescence in mycelia exhibited much more notable reduction of 72.39% and 72.14% (Fig. 6b) compared with the SNP group, separately. The content of superoxide anion (O2−) in mycelia was induced rapidly around 30 min of SNP treatment, reaching a highest value of 6.47 µmol/g FW (fresh weight) with time up to day 7, which was 59.14% higher than that of control (Fig. 6c). The change trends of hydrogen peroxide (H2O2) concentration were consistent with O2− production. The generation of H2O2 was strikingly increased from 9.12 to 11.75 µmol/g FW after 2–4-day treatment of SNP and then decreased on day 7–9, but it was still higher than those of the control group (Fig. 6d). As shown in Fig. 7, the activities and expression levels of three oxidoreductases were significantly stimulated by SNP. The most significant stimulation of enzyme activities of NOX, catalase (CAT) and superoxide dismutase (SOD) occurred on day 7, which were 157.71%, 85.49% and 64.71% higher than those of control, respectively. Accordingly, the transcriptional expression levels of NOX, CAT and SOD were activated on day 5 or 7, and the strongest induction effect appeared on day 7, about 3.71-, 2.90- and 4.21-fold of control, separately.
Effect of SNP treatment on ROS production in mycelium of Shiraia sp. S9. a Bright-field images (left) and fluorescence microscopy (right) of DCFH-DA-stained mycelia (400 ×). b ROS accumulation in mycelium after SNP treatment. Vc (0.01 mM) and DPI (5.00 μM) were added 30 min prior to SNP treatment. The contents of O2− c and H2O2 d in mycelium of S9 by SNP. The photos and contents of ROS were taken and detected after 2 h of SNP treatment. The SNP treatment was the same as specified in Fig. 3. The arrow represents the time of SNP addition. Values are mean ± SD from three independent experiments (*p < 0.05, **p < 0.01 vs. control and ##p < 0.01 vs. SNP)
Effects of SNP treatment on activities and expressions of NOX (a, b), CAT (c, d) and SOD (e, f) in Shiraia sp. S9 mycelium on day 1–9. NOX (comp2367_c0_seq1), CAT (comp15524_c0_seq1), SOD (CL8477Contig1). The SNP treatment was the same as specified in Fig. 3. The arrow represents the time of SNP addition. Values are mean ± SD from three independent experiments (*p < 0.05 and **p < 0.01 vs. control)
Discussion
SNP is a potent vasodilator used clinically to treat hypertensive emergencies and heart failure. In addition, it is widely used as nitric oxide donor in pharmacologic studies to investigate on the physiological roles of NO [14]. In plant cells or root cultures, SNP has been used as NO donor to elicit the production of important bioactive secondary metabolites such as anthocyanin and flavonol glycoside in Pisum sativum [30], and tropane alkaloids in hair roots culture of Hyoscyamus reticulatus [31]. More recently, the signal roles of NO in fungal developmental and metabolic biosynthesis have drawn more attention [32]. With the help of using NO donor SNP and NO scavengers, previous studies indicated that NO modulated the germination of C. coccodes conidia [21], hyphal elongation of Magnaporthe oryzae [33] and biosynthesis of fungal secondary metabolites such as styrylpyrone polyphenols, flavonoids and phenolics [62]. Meanwhile, the significance of gene expression differences was evaluated using the cut-off criteria of |foldchange|≥ 2 and p value < 0.05.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted using RNAprep pure Plant Kit (Tiangen, Bei**g, China) according to the instruction. The primer sequences of 18S ribosomal RNA (internal reference gene) and selected unigenes from NCBI database of SRR7293200-7,293,205 and PRJNA323638 were listed in Additional file 1: Table S9. The qRT-PCR was performed according to the method in our previous study [61]. And, the transcriptional expression levels of selected genes were calculated from cycle threshold values by using the 2−△△CT method described in detail by Zhang et al. [63].
Statistical analysis
Data analyses were carried out using Microsoft Excel and expressed as Mean ± Standard Deviation (SD). Student's t-test was applied for the comparison of the means between two groups. One-way analysis of variance (ANOVA) test was used for the comparison of the means among multiple groups. p < 0.05 is considered statistically significant.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its Additional files.
References
Zhen J, Di W. Novel therapeutic and diagnostic applications of hypocrellins and hypericins. Photochem Photobiol. 1995;61:529–39.
Dai DQ, Phookamsak R, Wijayawardene NN, Li WJ, Bhat DJ, Xu JC, et al. Bambusicolous fungi. Fungal Divers. 2017;82:1–105.
Kishi T, Tahara S, Taniguchi N, Tsuda M, Tanaka C, Takahashi S. New perylenequinones from Shiraia bambusicola. Planta Med. 1991;57:376–9.
Mulrooey CA, O’Brien EM, Morgan BJ, Kozlowski MC. Perylenequinones: isolation, synthesis, and biological activity. Eur J Org Chem. 2012;21:3887–904.
O’Brien EM, Morgan BJ, Mulrooney CA, Carroll PJ, Kozlowski MC. Perylenequinone natural products: total synthesis of hypocrellin A. J Org Chem. 2010;75:57–68.
Cai YJ, Liang XH, Liao XR, Ding YR, Sun J, Li XH. High-yield hypocrellin A production in solid-state fermentation by Shiraia sp. SUPER-H168. Appl Biochem Biotechnol. 2010;160:2275–86.
Yang HL, **ao C, Ma W, He G. The production of hypocrellin colorants by submerged cultivation of the medicinal fungus Shiraia bambusicola. Dyes Pigm. 2009;82:142–6.
Du W, Sun CL, Wang BG, Wang YM, Dong B, Liu JH, et al. Response mechanism of hypocrellin colorants biosynthesis by Shiraia bambusicola to elicitor PB90. AMB Expr. 2019;9:146.
Ma YJ, Zheng LP, Wang JW. Inducing perylenequinone production from a bambusicolous fungus Shiraia sp. S9 through co-culture with a fruiting body-associated bacterium Pseudomonas fulva SB1. Microb Cell Fact. 2019;18:121.
Lei XY, Zhang MY, Ma YJ, Wang JW. Transcriptomic responses involved in enhanced production of hypocrellin A by addition of Triton X-100 in submerged cultures of Shiraia bambusicola. J Ind Microbiol Biotechnol. 2017;44:1415–29.
Li XP, Ma YJ, Wang JW. Adding bamboo charcoal powder to Shiraia bambusicola preculture improves hypocrellin A production. Sustain Chem Pharm. 2019;14:100191.
Du W, Liang JD, Han YF, Yu JP, Liang ZQ. Nitric oxide mediates hypocrellin accumulation induced by fungal elicitor in submerged cultures of Shiraia bambusicola. Biotechnol Lett. 2015;37:153–9.
Li XP, Wang Y, Ma YJ, Wang JW, Zheng LP. Nitric oxide and hydrogen peroxide signaling in extractive Shiraia fermentation by Triton X-100 for hypocrellin A production. Int J Mol Sci. 2020;21:882.
Tuteja N, Chandra M, Tuteja R, Misra MK. Nitric oxide as a unique bioactive signaling messenger in physiology and pathophysiology. J Biomed Biotechnol. 2004;4:227–37.
Zhang B, Zheng LP, Wang JW. Nitric oxide elicitation for secondary metabolite production in cultured plant cells. Appl Microbiol Biotechnol. 2012;93:455–66.
Farouk S, Al-Huqail AA. Sodium nitroprusside application regulates antioxidant capacity, improves phytopharmaceutical production and essential oil yield of marjoram herb under drought. Ind Crops Prod. 2020;158:113034.
Wu CH, Tewari RK, Hahn EJ, Paek KY. Nitric oxide elicitation induces the accumulation of secondary metabolites and antioxidant defense in adventitious roots of Echinacea purpurea. J Plant Biol. 2007;50:636–43.
Zheng LP, Guo YT, Wang JW, Tan RX. Nitric oxide potentiates oligosaccharide- induced artemisinin production in Artemisia annua hairy roots. J Integr Plant Biol. 2008;50:49–55.
Wang JW, Zheng LP, Wu JY, Tan RX. Involvement of nitric oxide in oxidative burst, phenylalanine ammonia-lyase activation and taxol production induced by low-energy ultrasound in Taxus yunnanensis cell suspension cultures. Nitric Oxide. 2006;15:351–8.
Gong XY, Fu YP, Jiang DH, Li GQ, Yi XH, Peng YL. L-Arginine is essential for conidiation in the filamentous fungus Coniothyrium minitans. Fungal Genet Biol. 2007;44:1368–79.
Wang J, Higgins VJ. Nitric oxide has a regulatory effect in the germination of conidia of Colletotrichum coccodes. Fungal Genet Biol. 2005;42:284–92.
Baidya S, Cary JW, Grayburn WS, Calvo AM. Role of nitric oxide and flavohemoglobin homolog genes in Aspergillus nidulans sexual development and mycotoxin production. Appl Environ Microbiol. 2011;77:5524–8.
Zhao YX, ** Q, Xu Q, He MH, Ding JN, Dai YC, et al. Correlation of nitric oxide produced by an inducible nitric oxide synthase-like protein with enhanced expression of the phenylpropanoid pathway in Inonotus obliquus cocultured with Phellinus morii. Appl Microbiol Biotechnol. 2015;99:4361–72.
Gu L, Zhong X, Lian DH, Zheng YM, Wang HZ, Liu X. Triterpenoid biosynthesis and the transcriptional response elicited by nitric oxide in submerged fermenting Ganoderma lucidum. Process Biochem. 2017;60:19–26.
Sun CX, Ma YJ, Wang JW. Enhanced production of hypocrellin A by ultrasound stimulation in submerged cultures of Shiraia bambusicola. Ultrason Sonochem. 2017;38:214–24.
Wang JW, Zheng LP, Zhang B, Zou T. Stimulation of artemisinin synthesis by combined cerebroside and nitric oxide elicitation in Artemisia annua hairy roots. Appl Microbiol Biotechnol. 2009;85:285–92.
Yang HL, Wang Y, Zhang ZB, Yan RM, Zhu D. Whole-genome shotgun assembly and analysis of the genome of Shiraia sp. strain Slf14, a novel endophytic fungus producing huperzine A and hypocrellin A. Genome Announce. 2014;2:e00011-4.
Ren XY, Liu YX, Tan YM, Huang YH, Liu ZY, Jiang XL. Sequencing and functional annotation of the whole genome of Shiraia bambusicola. G3 Genes Genomes Genet. 2020;10:23–35.
Zhao N, Li D, Guo BJ, Tao X, Lin X, Yan SZ, et al. Genome sequencing and analysis of the hypocrellin-producing fungus Shiraia bambusicola S4201. Front Microbiol. 2020;11:643.
Ganjewala D, Bob S, Raghavendra AS. Sodium nitroprusside affects the level of anthocyanin and flavonol glycosides in pea (Pisum sativum L. cv Arkel) leaves. Acta Biol Szegediensis. 2008;52:301–5.
Khezerluo M, Hosseini B, Amiri J. Sodium nitroprusside stimulated production of tropane alkaloids and antioxidant enzymes activity in hairy root culture of Hyoscyamus reticulatus L. Acta Biol Hung. 2018;69:437–48.
Zhao YX, Lim JY, Xu JY, Yu JH, Zheng WF. Nitric oxide as a developmental and metabolic signal in filamentous fungi. Mol Microbiol. 2020;113:872–82.
Zhang Y, Shi HB, Liang S, Ning GA, Xu NC, Lu JP, et al. MoARG1, MoARG5,6 and MoARG7 involved in arginine biosynthesis are essential for growth, conidiogenesis, sexual reproduction, and pathogenicity in Magnaporthe oryzae. Microbiol Res. 2015;180:11–22.
Dong JF, Zhang M, Lu L, Sun LN, Xu MJ. Nitric oxide fumigation stimulates flavonoid and phenolic accumulation and enhances antioxidant activity of mushroom. Food Chem. 2012;135:1220–5.
Sun CX, Ma YJ, Wang JW. Improved hypocrellin A production in Shiraia bambusicola by light-dark shift. J Photochem Photobiol B Biol. 2018;182:100–7.
Ma YJ, Sun CX, Wang JW. Enhanced production of hypocrellin A in submerged cultures of Shiraia bambusicola by red light. Photochem Photobiol. 2019;95:812–22.
Du W, Liang ZQ, Zou X, Han YF, Liang JD, Yu JP, et al. Effects of microbial elicitor on production of hypocrellin by Shiraia bambusicola. Folia Microbiol. 2013;58:283–9.
Besson-Bard A, Pugin A, Wendehenne D. New insights into nitric oxide signaling in plants. Annu Rev Plant Biol. 2008;59:21–39.
Zheng LP, Zhang B, Zou T, Chen ZH, Wang JW. Nitric oxide interacts with reactive oxygen species to regulate oligosaccharide-induced artemisinin biosynthesis in Artemisia annua hairy roots. J Med Plants Res. 2010;4:758–66.
Iigusa H, Yoshida Y, Hasunuma K. Oxygen and hydrogen peroxide enhance light-induced carotenoid synthesis in Neurospora crassa. FEBS Lett. 2005;579:4012–6.
Ponts N, Pinson-Gadais L, Verdal-Bonnin MN, Barreau C, Richard-Forget F. Accumulation of deoxynivalenol and its 15-acetylated form is significantly modulated by oxidative stress in liquid cultures of Fusarium graminearum. FEMS Microbiol Lett. 2006;258:102–7.
Reverberi M, Gazzetti K, Punelli F, Scarpari M, Zjalic S, Ricelli A, et al. Aoyap1 regulates OTA synthesis by controlling cell redox balance in Aspergillus ochraceus. Appl Microbiol Biotechnol. 2012;95:1293–304.
Zhao N, Lin X, Qi SS, Luo ZM, Chen SL, Yan SZ. De novo transcriptome assembly in Shiraia bambusicola to investigate putative genes involved in the biosynthesis of hypocrellin A. Int J Mol Sci. 2016;17:311.
Hu JY, Sarrami F, Li H, Zhang GZ, Stubbs KA, Lacey E, et al. Heterologous biosynthesis of elsinochrome A sheds light on the formation of the photosensitive perylenequinone system. Chem Sci. 2019;10:1457–65.
Deng HX, Gao RJ, Liao XR, Cai YJ. Genome editing in Shiraia bambusicola using CRISPR-Cas9 system. J Biotechnol. 2017;259:228–34.
Li D, Zhao N, Guo BJ, Lin X, Chen SL, Yan SZ. Gentic overexpression increases production of hypocrellin A in Shiraia bambusicola S4201. J Microbiol. 2019;57:154–62.
Meraj TA, Fu JY, Raza MA, Zhu CY, Shen QQ, Xu DB, Wang Q. Transcriptional factors regulate plant stress responses through mediating secondary metabolism. Genes. 2020;11:346.
Beseli A, Amnuaykanjanasin A, Herrero S, Thomas E, Daub ME. Membrane transporters in self resistance of Cercospora nicotianae to the photoactivated toxin cercosporin. Curr Genet. 2015;61:601–20.
Ma YJ, Zheng LP, Wang JW. Bacteria associated with Shiraia fruiting bodies influence fungal production of hypocrellin A. Front Microbiol. 2019;10:2023.
Yao LY, Zhu YX, Jiao RH, Lu YH, Tan RX. Enhanced production of fumigaclavine C by ultrasound stimulation in a two-stage culture of Aspergillus fumigatus CY018. Bioresour Technol. 2014;159:112–7.
Turrion-Gomez JL, Benito EP. Flux of nitric oxide between the necrotrophic pathogen Botrytis cinerea and the host plant. Mol Plant Pathol. 2011;12:606–16.
Thevissen K, Terras FRG, Broekaert WF. Permeabilization of fungal membranes by plant defensins inhibits fungal growth. Appl Environ Microbiol. 1999;65:5451–8.
You BJ, Lee MH, Tien N, Lee MS, Hsieh HC, Tseng LH, et al. A novel approach to enhancing ganoderic acid production by Ganoderma lucidum using apoptosis induction. PLoS ONE. 2013;8:e53616.
Wu YX, Tiedemann AV. Impact of fungicides on active oxygen species and antioxidant enzymes in spring barley (Hordeum vulgare L.) exposed to ozone. Environ Pollut. 2002;116:37–47.
Pan WS, Zheng LP, Tian H, Li WY, Wang JW. Transcriptome responses involved in artemisinin production in Artemisia annua L under UV-B radiation. J Photochem Photobiol B Biol. 2014;140:292–300.
Aebi H. Catalase in vitro. Method Enzymol. 1984;105:121–6.
Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–74.
Tongul B, Tarhan L. The effect of menadione-induced oxidative stress on the in vivo reactive oxygen species and antioxidant response system of Phanerochaete chrysosporium. Process Biochem. 2014;49:195–202.
Lu CS, Ma YJ, Wang JW. Lanthanum elicitation on hypocrellin A production in mycelium cultures of Shiraia bambusicola is mediated by ROS generation. J Rare Earth. 2019;37:895–902.
Liu B, Bao JY, Zhang ZB, Yan RM, Wang Y, Yang HL, et al. Enhanced production of perylenequinones in the endophytic fungus Shiraia sp Slf14 by calcium/calmodulin signal transduction. Appl Microbiol Biotechnol. 2018;102:153–63.
Ma YJ, Lu CS, Wang JW. Effects of 5-azacytidine on growth and hypocrellin production of Shiraia bambusicola. Front Microbiol. 2018;9:2508.
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Map** and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5:621–8.
Zhang J, Zhu LY, Chen HY, Li M, Zhu XJ, Gao Q, et al. A polyketide synthase encoded by the gene An15g07920 is involved in the biosynthesis of ochratoxin A in Aspergillus niger. J Agric Food Chem. 2016;64:9680–8.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 82073955 and 81773696) and the Priority Academic Program Development of the Jiangsu Higher Education Institutes (PAPD).
Author information
Authors and Affiliations
Contributions
JWW and YJM conceived the study and participated in its design. YW, XPL and YJM undertook experiments and data analysis. YJM, XPL and YW drafted the manuscript. JWW supervised the research and revised the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Illumina RNA-Seq reads and de novo assembly statistics of Shiraia sp. S9 by SNP. Table S2. Summary statistics of unigene annotation numbers of Shiraia sp. S9 by SNP. Table S9. Primers and relevant information of reference and target genes. F: forward primer, R: reverse primer. Figure S1. Effect of SNP at 0.10 mM on the growth and development of Shiraia sp. S9 in submerged culture. Time profiles of fungal biomass (A) and average pellet diameters (B) during the production culture. Figure S2. Time profiles of pH (A) and residual sugar (B) in Shiraia sp. S9 submerged cultures with the addition of SNP at 0.10 mM. Figure S3. Chromatograms of PQs in mycelium of Shiraia sp. S9 treated by SNP (0.10 mM) on day 3 and cultivated for 8 days. Figure S4. Effect of SNP concentration on (A) hyphal growth, (B) HA content in mycelium and (C) the released HA in cultural broth of Shiraia sp. S9. The strain was treated by SNP (0.01–1.00 mM) on day 3. Figure S5. Effect of SNP addition time on (A) hyphal growth, (B) HA content in mycelium and (C) the released HA in cultural broth of Shiraia sp. S9. SNP (0.02 mM) was added on different cultural time (1–5 d). Figure S6. The length distribution of unigenes of Shiraia sp. S9 by SNP. Figure S7. Heat map of DEGs summary of Shiraia sp. S9 by SNP. The up/down-regulated DEGs were detected by comparing RNA-Seq data of the unigenes of case group to control group (up: ratio > 1, down: ratio < 1). Figure S8. The cellular component (CC), biological process (BP) and molecular function (MF) of gene ontology (GO) categories of the DEGs in Shiraia sp. S9 under SNP treatment at 0.02 mM. The arrow represents the time of SNP addition. Values are mean ± SD from three independent experiments (**p < 0.01 vs. control). Different letters above the bars mean significant differences (p < 0.05).
Additional file 2: Table S3.
Annotation of DEGs using NR, SWISS-Prot, KEGG, KOG and GO databases and their fold change with SNP treatment. Table S4. GO enrichment of Shiraia sp. S9 with SNP treatment. Table S5. Differentially expressed genes (DEGs) involved in hypocrellin biosynthesis of Shiraia sp. S9 under SNP treatment. Table S6. Differentially expressed genes (DEGs) involved in hypocrellin transport of Shiraia sp. S9 under SNP treatment. Table S7. Annotation of transcription factors in Shiraia sp. S9. Table S8. Differentially expressed genes (DEGs) involved in transcription factors of Shiraia sp. S9 under SNP treatment.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Ma, Y.J., Li, X.P., Wang, Y. et al. Nitric oxide donor sodium nitroprusside-induced transcriptional changes and hypocrellin biosynthesis of Shiraia sp. S9. Microb Cell Fact 20, 92 (2021). https://doi.org/10.1186/s12934-021-01581-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-021-01581-8