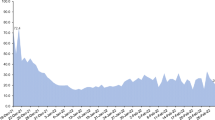
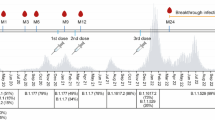
Abstract
Background
Although COVID-19 vaccines and their booster regimens protect against symptomatic infections and severe outcomes, there is limited evidence about their protection against asymptomatic and symptomatic infections in real-world settings, particularly when considering that the majority of SARS-CoV-2 Omicron infections were asymptomatic. We aimed to assess the effectiveness of the booster dose of inactivated vaccines in mainland China, i.e., Sinopharm (BBIBP-CorV) and Sinovac (CoronaVac), against Omicron infection in an Omicron BA.5 seeded epidemic.
Methods
Based on an infection-naive but highly vaccinated population in Urumqi, China, the study cohort comprised all 37,628 adults who had a contact history with individuals having SARS-CoV-2 infections, i.e., close contacts, between August 1 and September 7, 2022. To actively detect SARS-CoV-2 infections, RT-PCR tests were performed by local authorities on a daily basis for all close contacts, and a testing-positive status was considered a laboratory-confirmed outcome. The cohort of close contacts was matched at a ratio of 1:5 with the fully vaccinated (i.e., 2 doses) and booster vaccinated groups (i.e., 3 doses) according to sex, age strata, calendar date, and contact settings. Multivariate conditional logistic regression models were adopted to estimate the marginal effectiveness of the booster dose against Omicron BA.5 infection after adjusting for confounding variables. Subgroup analyses were performed to assess vaccine effectiveness (VE) in different strata of sex, age, the time lag from the last vaccine dose to exposure, and the vaccination status of the source case. Kaplan–Meier curves were employed to visualize the follow-up process and testing outcomes among different subgroups of the matched cohort.
Findings
Before matching, 37,099 adult close contacts were eligible for cohort enrolment. After matching, the 2-dose and 3-dose groups included 3317 and 16,051 contacts, and the proportions with Omicron infections were 1.03% and 0.62% among contacts in the 2-dose and 3-dose groups, respectively. We estimated that the adjusted effectiveness of the inactivated booster vaccine versus 2 doses against Omicron infection was 35.5% (95% CI 2.0, 57.5). The booster dose provided a higher level of protection, with an effectiveness of 60.2% (95% CI 22.8, 79.5) for 15–180 days after vaccination, but this VE decreased to 35.0% (95% CI 2.8, 56.5) after 180 days. Evidence for the protection of the booster dose was detected among young adults aged 18–39 years, but was not detected for those aged 40 years or older.
Interpretation
The receipt of the inactivated vaccine booster dose was associated with a significantly lower Omicron infection risk, and our findings confirmed the vaccine effectiveness (VE) of booster doses against Omicron BA.5 variants. Given the rapid evolution of SARS-CoV-2, we highlight the importance of continuously monitoring the protective performance of vaccines against the genetic variants of SARS-CoV-2, regardless of existing vaccine coverage.
Similar content being viewed by others
Introduction
Although vaccine program is an effective strategy in fighting against the COVID-19 pandemic [1,2,3], the current global predominance of SARS-CoV-2 Omicron variants continuously challenges vaccine-induced protection, which was recognized by the World Health Organization (WHO) as one of the major public health concerns. Evidence from recent studies also suggested that the immunity generated by vaccination may wane over time [4,5,6,7,8,9,10], and Omicron variants were associated with increased transmissibility and immune escape ability [11,12,13,14,15]. The booster dose of vaccines was used to enhance immunity levels [16, 17], and was reported to provide relatively high protection against symptomatic to severe COVID-19 outcomes, including hospitalization, need for intensive care, and death [18,19,20,21,22,23,24]. Most existing estimates of vaccine effectiveness (VE) against Omicron infections have focused on various mRNA vaccines, including mRNA-1273 and BNT162b2, or adenovirus vector vaccines, such as ChAdOx1-nCoV-19 [24,25,26,27,28]. The COVID-19 vaccines received by almost all vaccinees in mainland China were Sinopharm (BBIBP-CorV) and Sinovac (CoronaVac) inactivated COVID-19 vaccines, mainly BBIBP-CorV in Urumqi city (the city where the cohort was recruited in our study). Although the efficacy of inactivated vaccines was assessed in phase III clinical trials [17, 29], the real-world evidence of the effectiveness of inactivated vaccines remains largely unassessed [30, 31], especially considering the challenges caused by genetic variants of SARS-CoV-2, and immunity waning after vaccination.
Considering that the majority of Omicron infections may not progress to pneumonia and some of them are subclinical [32, 33], real-world evidence of VE in preventing mild and asymptomatic Omicron infections is generally lacking, but it is important for develo** herd immunity. The evaluation of VE against asymptomatic and mild infections is potentially challenging because infections without identifiable symptoms were less likely to be ascertained. As such, VE estimates under common study designs, including test-negative designs, could bias toward more severe clinical conditions or subgroups of populations with relatively high test-seeking behaviors [34], which may fail to be a fair representation of all infections. To our knowledge, there is only one study that assessed the effectiveness of inactivated vaccines against (asymptomatic and symptomatic) Omicron BA.2 infection in Hong Kong by using a cohort design, and the cohort was collected from participants randomly selected from the general population [35]. However, the contact tracing information was uninvestigated in their study, such that the determinants that contribute to secondary transmission, e.g., contact settings or the vaccination status of source cases, and then the downstream infection of close contacts remained unadjusted for or studied. In addition, the ongoing (as of December 2022) COVID-19 pandemic was dominated by Omicron BA.5 and its genetic sublineages [36], which have replaced Omicron BA.2 globally since the middle of 2022; thus, updating the VE against the (most recent) circulation SARS-CoV-2 variants may inform the risk assessment of current COVID-19 situations.
In this study, we assessed the effectiveness of the booster dose of inactivated vaccines against asymptomatic and symptomatic Omicron BA.5 infections in a well-traced cohort including all documented adult COVID-19 close contacts from August 1 to September 7, 2022. This cohort was collected from an infection-naive population with relatively high vaccination coverage in Urumqi, the capital and largest city in the ** severe clinical conditions of COVID-19, and the VE among the elderly is also far less than that for young people [61,62,63].
We reported that the VE was high when both infected individuals and their contacts were vaccinated with three doses. This finding was compatible with the first point of our findings that the overall effectiveness of booster vaccination against Omicron variant infection outperformed that of the primary series of two-dose vaccination. Unlike other places outside mainland China, quarantine and lockdown have been implemented for a longer time as a pandemic intervention strategy in the ** herd immunity. The assessment of vaccine effectiveness (VE) against asymptomatic Omicron infections generally lacking because the ascertainment of asymptomatic or mild infections was potentially challenging, despite the majority of Omicron infections being asymptomatic. Thus, VE estimates under common study designs, including test-negative designs, could bias toward more severe clinical conditions or subgroups of populations with high test-seeking behaviors. As of December 2022, we found 1 peer-reviewed cohort study based on the population in Hong Kong, China that assessed the VE of BNT162b2 and CoronaVac against asymptomatic Omicron BA.2 infection using real-world individual-level data. Owning to the previous “zero-COVID” policy in mainland China, COVID-19 was at a relatively low level before December 2022, and thus no VE estimate of BBIBP-CorV against Omicron infection in mainland China was published. To our knowledge, to date (December 2022), no study has reported the VE of BBIBP-CorV booster against Omicron BA.5 asymptomatic infection.
Added value of this study: in an Omicron BA.5 seeded outbreak in Urumqi, the capital and largest city in **njiang Uygur Autonomous Region of China, we identified 37,628 adult close contacts of COVID-19 with 2 or 3 doses of inactivated vaccines before exposure from August 1 to September 7, 2022. After matching for baseline conditions, we assessed the effectiveness of inactivated COVID-19 vaccines (mainly BBIBP-CorV) against Omicron infection, regardless of symptoms, in a real-world setting. The overall VE of booster dose versus 2-dose against Omicron BA.5 infection was 35.5% (95% CI 2.0, 57.5), with an effectiveness of 60.2% (95% CI 22.8, 79.5) for 15–180 days after vaccination, but decreased to 35.0% (95% CI 2.8, 56.5) after 180 days. These findings were the first VE estimates against SARS-CoV-2 Omicron BA.5 infection in mainland China.
Implications of all the available evidence: moderate but significant protective effects against asymptomatic and symptomatic Omicron BA.5 infection were found for the booster doses of inactivated vaccine. The VE estimates were important contributions to informing vaccination policy in places where vaccine coverage remains low or inactivated vaccines were in-use. Thus, it is important to assess the vaccine performance against emerging genetic variants of SARS-CoV-2, as they evolved, regardless of the background vaccine coverage.
Data availability
The original database containing confidential patient information cannot be made publicly available. The anonymized data used in this study were available based on reasonable request to the corresponding authors.
References
Hodgson SH, et al. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis. 2021;21:E26–35.
Dagotto G, Yu JY, Barouch DH. Approaches and challenges in SARS-CoV-2 vaccine development. Cell Host Microbe. 2020;28:364–70.
Dai LP, Gao GF. Viral targets for vaccines against COVID-19. Nat Rev Immunol. 2021;21:73–82.
Rennert L, Ma Z, McMahan CS, Dean D. Effectiveness and protection duration of Covid-19 vaccines and previous infection against any SARS-CoV-2 infection in young adults. Nat Commun. 2022;13:3946.
Tartof SY, et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–16.
Chemaitelly H, et al. Waning of BNT162b2 vaccine protection against SARS-CoV-2 infection in Qatar. N Engl J Med. 2021;385:E83–E83.
Levin EG, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385:E84.
Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science. 2022;375:331.
Levine-Tiefenbrun M, et al. Viral loads of Delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nat Med. 2021;27:2108.
Pouwels KB, et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021;27:2127.
Liu Y, Rocklov J. The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus. J Travel Med. 2021;28:3.
Liu Y, Rocklov J. The effective reproductive number of the Omicron variant of SARS-CoV-2 is several times relative to Delta. J Travel Med. 2022;29:4.
Chen Z, et al. Epidemiological characteristics and transmission dynamics of the outbreak caused by the SARS-CoV-2 Omicron variant in Shanghai, China: a descriptive study. Lancet Reg Health West Pac. 2022;29: 100592.
Hachmann NP, et al. Neutralization escape by SARS-CoV-2 omicron subvariants BA.2.12.1, BA.4, and BA.5. N Engl J Med. 2022;387:86–8.
Cao Y, et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by omicron infection. Nature. 2022;608:593–602.
Liu XX, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398:856–69.
Organization, W.H. Recommendation for an emergency use listing of Covid-19 vaccine (Vero cell), inactivated—submitted by Sinovac. Geneva. (https://extranet.who.int/pqweb/sites/default/files/documents/SINOVAC_TAG_PEG_REPORT_EUL-Final28june2021.pdf. Accessed 28 June 2021.
Sheikh A, et al. Severity of omicron variant of concern and effectiveness of vaccine boosters against symptomatic disease in Scotland (EAVE II): a national cohort study with nested test-negative design. Lancet Infect Dis. 2022;22(7):959–66.
Lauring AS, et al. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022;376: e069761.
Cao L, et al. Rapid evaluation of COVID-19 vaccine effectiveness against symptomatic infection with SARS-CoV-2 variants by analysis of genetic distance. Nat Med. 2022;28:1715–22.
Chemaitelly H, et al. Duration of mRNA vaccine protection against SARS-CoV-2 Omicron BA. 1 and BA. 2 subvariants in Qatar. Nat Commun. 2022;13:1–12.
Tartof SY, et al. Durability of BNT162b2 vaccine against hospital and emergency department admissions due to the omicron and delta variants in a large health system in the USA: a test-negative case–control study. Lancet Respir Med. 2022;10(7):689–99.
Kirsebom FC, et al. COVID-19 vaccine effectiveness against the omicron (BA. 2) variant in England. Lancet Infect Dis. 2022;22(7):931–3.
Fleming-Dutra KE, et al. Association of prior BNT162b2 COVID-19 vaccination with symptomatic SARS-CoV-2 infection in children and adolescents during omicron predominance. JAMA. 2022;327(22):2210–9.
McMenamin ME, et al. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect Dis. 2022;22:1435–43.
Gazit S, et al. BNT162b2 mRNA vaccine effectiveness given confirmed exposure: analysis of household members of coronavirus disease 2019 patients. Clin Infect Dis. 2022;75:e734–40.
Layan M, et al. Impact of BNT162b2 vaccination and isolation on SARS-CoV-2 transmission in Israeli households: an observational study. Am J Epidemiol. 2022;191:1224–34.
Mousa M, et al. Similar effectiveness of the inactivated vaccine BBIBP-CorV (Sinopharm) and the mRNA vaccine BNT162b2 (Pfizer-BioNTech) against COVID-19 related hospitalizations during the Delta outbreak in the UAE. J Travel Med. 2022;29: taac036.
Tanriover MD, et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet. 2021;398:213–22.
Al Kaabi N, et al. Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infection in adults: a randomized clinical trial. JAMA. 2021;326:35–45.
Jara A, et al. Effectiveness of an inactivated SARS-CoV-2 vaccine in Chile. N Engl J Med. 2021;385:875–84.
Garrett N, et al. High asymptomatic carriage with the omicron variant in South Africa. Clin Infect Dis. 2022;75:E289–92.
Yu W, et al. Proportion of asymptomatic infection and nonsevere disease caused by SARS-CoV-2 Omicron variant: a systematic review and analysis. J Med Virol. 2022;94:5790–801.
Kuitunen I, Uimonen M, Seppälä SJ, Ponkilainen VT. COVID-19 vaccination status and testing rates in Finland—a potential cause for bias in observational vaccine effectiveness analysis. Influenza Other Respir Viruses. 2022;16(5):842–5.
Tsang NNY, So HC, Cowling BJ, Leung GM, Ip DKM. Effectiveness of BNT162b2 and CoronaVac COVID-19 vaccination against asymptomatic and symptomatic infection of SARS-CoV-2 omicron BA. 2 in Hong Kong: a prospective cohort study. Lancet Infect Dis. 2022;23(4):421–34.
Shu Y, McCauley J. GISAID: global initiative on sharing all influenza data—from vision to reality. Eurosurveillance. 2017;22:30494.
Agency, T.X.N. The safety and effectiveness of COVID-19 vaccines in China—the Joint prevention and control mechanism of the State Council answers questions on vaccination. http://www.gov.cn/govweb/xinwen/2022-07/23/content_5702572.htm. Accessed 23 July 2022.
O’Toole Á, Pybus OG, Abram ME, Kelly EJ, Rambaut A. Pango lineage designation and assignment using SARS-CoV-2 spike gene nucleotide sequences. BMC Genom. 2022;23:1–13.
Chadeau-Hyam M, et al. SARS-CoV-2 infection and vaccine effectiveness in England (REACT-1): a series of cross-sectional random community surveys. Lancet Respir Med. 2022;10:355–66.
Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat-Simul Comput. 2009;38:1228–34.
Flury BK, Riedwyl H. Standard distance in univariate and multivariate analysis. Am Stat. 1986;40:249–51.
Jackson ML, Nelson JC. The test-negative design for estimating influenza vaccine effectiveness. Vaccine. 2013;31:2165–8.
Bond HS, Sullivan SG, Cowling BJ. Regression approaches in the test-negative study design for assessment of influenza vaccine effectiveness. Epidemiol Infect. 2016;144:1601–11.
Cowling BJ, et al. Incidence of influenza virus infections in children in Hong Kong in a 3-year randomized placebo-controlled vaccine study, 2009–2012. Clin Infect Dis. 2014;59:517–24.
Sun K, et al. Rapidly shifting immunologic landscape and severity of SARS-CoV-2 in the Omicron era in South Africa. Nat Commun. 2023;14:246.
Baksh RA, Strydom A, Pape SE, Chan LF, Gulliford MC. Susceptibility to COVID-19 diagnosis in people with down syndrome compared to the general population: matched-cohort study using primary care electronic records in the UK. J Gen Intern Med. 2022;37:2009–15.
Calvert C, et al. A population-based matched cohort study of major congenital anomalies following COVID-19 vaccination and SARS-CoV-2 infection. Nat Commun. 2023;14:107.
Kaplan EL. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81.
R, R.C.T. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/.
Ho D, Imai K, King G, Stuart EA. MatchIT: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011. https://doi.org/10.18637/jss.v042.i08.
Organization, W.H. Coronavirus disease (covid-19): variants of SARS-COV-2. World Health Organization. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-%28covid-19%29-variants-of-sars-cov-2?gclid=CjwKCAiAhqCdBhB0EiwAH8M_GhPGuQ9dTNKI30G41zZGVATx3uZdswb8GBOq4CFvZP02Uk1TJJt_hRoCTMYQAvD_BwE. Accessed 25 Dec 2022.
Chicago, N. Omicron symptoms: here’s how they differ from other variants. (https://www.nbcchicago.com/news/local/omicron-symptoms-heres-how-they-differ-from-other-variants/2723960. Accessed 7 Jan 2022.
Huang Z, et al. Effectiveness of inactivated and Ad5-nCoV COVID-19 vaccines against SARS-CoV-2 Omicron BA. 2 variant infection, severe illness, and death. BMC Med. 2022;20:400.
Modelling, W.C.C.F.I. Growth, population distribution and immune escape of Omicron in England. in Report 49. 2022. https://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-19/report-49-omicron.
Tseng HF, et al. Effectiveness of mRNA-1273 against SARS-CoV-2 Omicron and Delta variants. Nat Med. 2022;28(5):1063–71.
Wang X, Zein J, Ji X, Lin D-Y. Impact of vaccination, prior infection, and therapy on delta and omicron variants. medRxiv. 2022. https://doi.org/10.1101/2022.03.24.22272901.
Ranzani OT, et al. Effectiveness of an inactivated Covid-19 vaccine with homologous and heterologous boosters against omicron in Brazil. medRxiv. 2022. https://doi.org/10.1101/2022.03.30.22273193.
Andeweg SP, et al. Protection of COVID-19 vaccination and previous infection against Omicron BA.1, BA.2 and Delta SARS-CoV-2 infections. Nat Commun. 2022;13:9.
Weekly, C.C. Promote covid-19 vaccination for older adults in China. 2022. https://doi.org/10.46234/ccdcw2022.173.
University, C.f.s.s.a.e.a.J.H. COVID-19 dashboard. https://coronavirus.jhu.edu/map.html. Accessed 25 Dec 2022.
Arregoces-Castillo L, et al. Effectiveness of COVID-19 vaccines in older adults in Colombia: a retrospective, population-based study of the ESPERANZA cohort. Lancet Healthy Longev. 2022;3:e242–52.
Nanishi E, Levy O, Ozonoff A. Waning effectiveness of SARS-CoV-2 mRNA vaccines in older adults: a rapid review. Hum Vaccines Immunother. 2022;18:2045857.
Emani VR, et al. Increasing SARS-CoV2 cases, hospitalizations, and deaths among the vaccinated populations during the Omicron (B.1.1.529) variant surge in UK. medRxiv. 2022. https://doi.org/10.1101/2022.06.28.22276926.
Region, T.G.o.t.H.K.S.A. COVID-19 vaccination programme. https://www.ovidvaccine.gov.hk/en/programme. Accessed 7 June 2022.
Prevention, U.C.f.D.C.a. Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the United States. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html. Accessed 20 May 2022.
Acknowledgements
We thank all participants in this study for their cooperation in disease surveillance and control measure. We also thank healthcare professionals, caregiver partners, and public health practitioners for their contributions to the community.
Funding
This study was supported by Natural Science Foundation of **njiang (Grant No. 2021D01C268), Collaborative Research Fund (Grant No. C7123-20G) of the Research Grants Council (RGC) of Hong Kong, China, National Natural Science Foundation of China (Grant Nos. 12171192, 12071173, 11961071), and the Youth Science and Technology Innovation Talent of the Tianshan Talent Training Program in **njiang, China (Grant No. 2022TSYCCX0099).
Author information
Authors and Affiliations
Contributions
Conceptualization: SZ and KW. Methodology: SZ, TZ, YL and ZG. Software: KW, TZ and YL. Validation: ZG and SZ. Formal analysis: KW, MT and ZT. Investigation: KW, TZ, YL and SZ. Resources: YL, YC and KW. Data curation: JW, SL and XF. Writing—original draft: TZ, YZ, ZG and YL. Writing—review and editing: SS, ZT, MT, JW, SL, XF, WW, YC, GL, XL, DH and SZ. Visualization: YL, TZ and ZG. Supervision: KW and SZ. Project administration: KW, WW and YL. Funding acquisition: TZ, WW, YC, DH and KW. All authors critically read the manuscript and gave final approval for publication.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The collection of specimens, epidemiological and clinical data for SARS-CoV-2 infected individuals and their close contacts is a part of a continuing public health investigation of COVID-19 outbreaks, ruled in the Protocol on the Prevention and Control of COVID-19 by the National Health Commission of the People’s Republic of China, which was exempt from ethical approval (i.e., institutional review board assessment). This study was approved by the institutional ethics committee of **njiang Medical University (IRB No. XJYKDXR20221001001). Individual verbal consent was obtained when collecting personal information and human samples by governmental healthcare professionals in the field.
Competing interests
All authors declared no competing interests. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zeng, T., Lu, Y., Zhao, Y. et al. Effectiveness of the booster dose of inactivated COVID-19 vaccine against Omicron BA.5 infection: a matched cohort study of adult close contacts. Respir Res 24, 246 (2023). https://doi.org/10.1186/s12931-023-02542-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12931-023-02542-y