Abstract
Background
Metazoan guts are in permanent contact with microbial communities. However, the host mechanisms that have developed to manage the dynamic changes of these microorganisms and maintain homeostasis remain largely unknown.
Results
Serotonin (5-hydroxytryptamine [5-HT]) was found to modulate gut microbiome homeostasis via regulation of a dual oxidase (Duox) gene expression in both Bactrocera dorsalis and Aedes aegypti. The knockdown of the peripheral 5-HT biosynthetic gene phenylalanine hydroxylase (TPH) increased the expression of Duox and the activity of reactive oxygen species, leading to a decrease in the gut microbiome load. Moreover, the TPH knockdown reduced the relative abundance of the bacterial genera Serratia and Providencia, including the opportunistic pathogens, S. marcescens and P. alcalifaciens in B. dorsalis. Treatment with 5-hydroxytryptophan, a precursor of 5-HT synthesis, fully rescued the TPH knockdown-induced phenotype.
Conclusions
The findings reveal the important contribution of 5-HT in regulating gut homeostasis, providing new insights into gut–microbe interactions in metazoans.
Similar content being viewed by others
Background
The monoamine serotonin (5-hydroxytryptamine [5-HT]) is a neurotransmitter in the animal brain. It regulates a wide variety of processes in most invertebrates and vertebrates, such as feeding [1], aggression [2], learning and memory [3] in Drosophila; mood, behavior, sleep cycles, and appetite in humans [4]. Serotonin is also an important regulatory factor outside of the central nervous system in immune signaling [5]. In mammals, more than 90% of the 5-HT is biosynthesized in the gut, and the gut-derived 5-HT regulates diverse functions [6]. For example, 5-HT interacts with microbiota in the mammalian gut. Elevated levels of intestinal lumenal 5-HT increase the relative abundance of spore-forming members of the gut microbiota [7], which in turn can promote host 5-HT biosynthesis [8, 9]. However, the precise mechanisms by which 5-HT modulates gut microbiome remains to be determined.
The metazoan intestine harbors numerous microbial communities that influence multiple aspects of host physiology, such as immunity, nutrition, and development [10, 11]. The quantity and composition of gut microbial communities may fluctuate dynamically in response to host diet, physical activities, and environmental conditions [12, 13]. Therefore, gut epithelia must tolerate a certain amount of proliferation of commensal microbes in order for beneficial gut–microbe interactions to occur. In addition, the gut needs to evade many types of noncommensal microorganisms, including foodborne microorganisms and pathogens. To maintain the intestinal microbiome homeostasis, finely tuned immune systems have evolved in metazoan gut. For example, Drosophila gut immune systems are primarily dual oxidase (Duox)-dependent reactive oxygen species (ROS) production [14, 15] and an immune deficiency (IMD) pathway [16].
Dipteran insects, such as Drosophila, share common aspects of gut–microbe interactions and gut physiology with many other metazoans [17]. Because of the relative simplicity of the insect intestinal system, examining the interactions between serotonin and gut microbiome homeostasis may be easier in insects. In recent years, insect gut–microbe interactions have attracted increasing attention. Gut symbionts mediate insecticide resistance in the agricultural pest Bactrocera dorsalis [Full size image
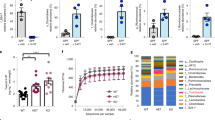
Then, the effect of the elevated level of 5-HT on the gut microbiome was evaluated. The gut bacterial population was investigated using both a culture-independent method (16S rRNA quantification by real-time PCR) and colony-forming unit counts (cultivable bacteria). With the 5-HTP treatment, the microbiome load increased by approximately twofold (Fig. 1b). The cultivable bacteria in 5-HTP-treated flies also increased significantly, compared with those in the control flies (Fig. 1c, d).
Whether the increase in serotonin changed the composition of the gut microbial community in B. dorsalis was also determined. The midguts and hindguts were isolated from flies for bacterial 16S rDNA sequencing. Treatment with 5-HTP affected the gut bacteria composition at the genus level (Additional file 1: Fig. S2). We focus specifically on Serratia and Providencia, because species of these two genera are insect opportunistic pathogens [21, 22]. Intriguingly, the abundance of Serratia and Providencia, which mainly inhabited the midgut and hindgut, respectively, elevated after 5-HTP treatment (Fig. 1e, f). The relative abundance of the opportunistic pathogens Serratia marcescens (Otu000010) and Providencia alcalifaciens (Otu000007) increased significantly in 5-HTP-treated flies (Additional file 1: Fig. S3a–c). The increased burdens of S. marcescens and P. alcalifaciens were confirmed by RT-qPCR (Fig. 1g, h).
Because of the increase in load of opportunistic pathogens in the fly gut, the effect of the 5-HTP treatment on fly survival was tested. With the oral introduction of 5-HTP, fly mortality was 100% within 9 days (Fig. 1i). While the mortality rate of the axenic flies in the presence of 5-HTP ingestion was about 10% (Fig. 1i). Then, flies were treated with the S. marcescens and P. alcalifaciens isolated from the gut of B. dorsalis. In the flies that ingested S. marcescens, 100% mortality occurred within 11 days (Fig. 1j). By contrast, the survival rate of flies fed P. alcalifaciens was 85% over 11 days (Fig. 1j). These results indicated that the 5-HTP-induced disturbance of gut microbiota decreased host survival.
Serotonin contributes to the maintenance of gut microbiota in B. dorsalis
On the basis of the above results, 5-HT was hypothesized to mediate insect gut microbiome homeostasis. Tryptophan hydroxylase is the rate-limiting enzyme for 5-HT biosynthesis in insects, and the different isoenzymes TPH and TRH mediate non-neuronal and neuronal 5-HT biosynthesis, respectively [23, 24]. The transcripts of both TPH and TRH were detected in the B. dorsalis intestinal tract (Fig. 2a), although the mRNA level of TPH was much higher than that of TRH (Fig. 2b). In addition, more TPH transcripts were expressed in the hindgut than in the midgut (Fig. 2b).
BdTPH contributes to the maintenance of gut microbiota in B. dorsalis. a Expression of TPH and TRH in the B. dorsalis gut. RpL32 was used as an internal reference gene. b Relative expression levels of BdTPH and BdTRH in the midgut and hindgut of B. dorsalis (n = 3). c Gut BdTPH silencing efficiency in B. dorsalis at 96 h postinjection with 2.0 μg of dsGFP or dsBdTPH. Data were normalized to expression levels in dsGFP-treated flies. d Effect of BdTPH knockdown on gut 5-HT level by HPLC detection post dsRNA injection. e–g Effect of BdTPH silencing on gut total bacterial load by e 16S rRNA quantification using real-time PCR and f, g colony-forming unit assay. h Relative abundance of the genera Serratia and Providencia postinjection of dsBdTPH and dsGFP by 16S rDNA sequencing. i BdTPH knockdown decreased the load of S. marcescens and j P. alcalifaciens in the gut of B. dorsalis. To restore the 5-HT level, BdTPH-silenced flies were treated with 1-mM 5-HTP by feeding after dsRNA injection. Data were analyzed using two-tailed unpaired t-test. Error bars indicate ± s.e.m.; *p < 0.05, **p < 0.01, ***p < 0.001. All results represent at least two independent experiments
To validate the role of TPH in controlling the gut microbiome, flies were injected with BdTPH double-stranded RNA (dsRNA) to silence the expression of the BdTPH gene. Flies treated with green fluorescent protein (GFP) dsRNA were the controls. Strong knockdown effect, approximately 97%, was observed post RNA-interference (RNAi) treatment (Fig. 2c). Compared with the GFP dsRNA-treated flies, the flies treated with BdTPH dsRNA had decreased levels of 5-HT in the gut, whereas treatment with 1-mM 5-HTP recovered the level of 5-HT (Fig. 2d). The diminished expression of BdTPH markedly inhibited the proliferation of intestinal tract bacteria (Fig. 2e–g). However, treatment with 5-HTP fully rescued the ds-BdTPH-induced phenotype (Fig. 2e–g). BdTPH dsRNA treatment changed the composition of the gut microbial community (Additional file 1: Fig. S4). Compared with the GFP dsRNA-treated controls, the relative abundance of the genus Serratia and Providencia decreased significantly after ds-BdTPH treatment (Fig. 2h and S4). However, by treating BdTPH-silenced flies with 1-mM 5-HTP, the reduction reversed dramatically (Fig. 2h, Additional file 1: Fig. S4). The abundances of S. marcescens and P. alcalifaciens in the B. dorsalis intestinal tract were then determined, and the loads of both species of bacteria decreased significantly, compared with those of the controls (Fig. 2i, j).
Next, the role of TRH in the maintenance of commensal gut microorganisms in B. dorsalis was examined. Intestinal tract BdTRH transcripts decreased significantly post RNAi treatment (Additional file 1: Fig. S5a). The ds-BdTRH silencing reduced midgut BdTRH mRNA levels but did not affect the amount of gut bacteria (Fig. S5a and b). There were no significant differences in the load of S. marcescens in the B. dorsalis intestinal tract between ds-BdTRH-treated flies and ds-GFP-treated controls (Additional file 1: Fig. S5c).
Collectively, the results indicated that the 5-HT controlled B. dorsalis gut microbiome homeostasis mainly via the peripheral serotonin biosynthetic enzyme TPH.
Serotonin regulates gut microbiome homeostasis by controlling Duox expression in B. dorsalis
In Drosophila, two mechanisms that maintain gut microbiome homeostasis are Duox-dependent ROS [14, 15] and IMD pathway-produced antimicrobial peptides (AMPs) [16]. To investigate the molecular mechanism by which 5-HT regulates gut microbiome proliferation, the effect of 5-HT on ROS production and AMP expression was determined.
In Drosophila intestine, ROS are produced and regulated by Duox [15] or nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox) genes [25]. Only one Duox gene and one Nox gene were identified in the genome of B. dorsalis, consistent with the findings in Drosophila melanogaster [15]. Compared with controls, the mRNA level of intestinal tract BdDuox in 5-HTP-treated flies was significantly downregulated by approximately twofold. However, 5-HTP had no effect on BdNox expression in conventionally colonized flies (Fig. 3a). To exclude the possibility that Duox has been activated by some microbiota, axenic flies were generated with antibiotics. The 5-HTP decreased BdDuox expression was observed in axenic fly guts (Fig. 3a). The reduced expression of BdDuox in 5-HTP-treated flies was consistent with the weaker ROS signal (Fig. 3b) and lower hydrogen peroxide (H2O2) production in the intestinal tract (Fig. 3c) of both conventionally colonized and axenic flies. Next, the regulation of BdDuox in the gut of BdTPH-silenced flies was validated by qPCR. Strong knockdown effect of BdTPH was observed post RNAi treatment in axenic adult B. dorsalis (Additional file 1: Fig. S6). The mRNA level of BdDuox was significantly upregulated post BdTPH silencing, compared with the GFP dsRNA-treated controls (Fig. 3d). In axenic flies, 5-HTP recovered BdDuox expression post BdTPH silencing (Fig. 3d). Consistent with this finding, ROS activity, as measured by dihydroethidium (DHE) (Fig. 3e) and H2O2 assay (Fig. 3f), increased consistently in the gut of BdTPH-silenced flies. The 5-HTP restored the ROS activity of both conventionally colonized and axenic flies post ds-BdTPH treatment (Fig. 3e, f). The knockdown of the BdTRH gene had no significant effect on BdDuox expression (Additional file 1: Fig. S7).
Serotonin controls gut BdDuox expression in B. dorsalis. a–c Effects of 5-HTP treatment on gut BdDuox and BdNox expression (a) and ROS activity (b, c) in conventionally colonized and axenic flies. ROS activity was measured by DHE (b) and H2O2 assay (c). Scale bars in b and e represent 1000 μm. Data of H2O2 level were normalized to controls. d–f Regulation of both BdDuox expression (d) and ROS activity (e, f) in the guts of conventionally colonized and axenic flies post dsBdTPH treatment. ROS activity was detected at 96 h post dsRNA injection. Data of H2O2 level were normalized to dsGFP-treated controls. To restore the 5-HT level, BdTPH-silenced flies were treated with 1-mM 5-HTP by feeding after dsRNA injection. g–i Gut BdDuox silencing efficiency determination in B. dorsalis by real-time PCR (g) and ROS activity (h, i). ROS activity was detected by DHE (h) and H2O2 assay (i) at 24 h post dsRNA injection. Scale bars in h represent 1000 μm. Data of H2O2 level were normalized to dsGFP-treated controls. j–m Effect of BdDuox silencing on gut total bacterial load (j) and the burden of S. marcescens (k) and P. alcalifaciens (m) at 24 h post dsRNA injection. Two-tailed unpaired t-test was performed in a, c, d, g, i, j, k, and m; one-way ANOVA followed by Tukey’s multiple comparison test for f. Conv, conventionally colonized flies; Axenic, axenic flies. Error bars indicate ± s.e.m.; *p < 0.05, **p < 0.01, ***p < 0.001. Results represent at least two independent experiments
To validate the role of ROS in the control of gut bacteria, BdDuox expression was silenced by dsRNA injection. The ds-BdDuox silencing reduced intestinal tract BdDuox mRNA levels by 54% at 24 h post injection (Fig. 3g). Compared with the GFP dsRNA-treated controls, a weaker ROS signal (Fig. 3h) and lower H2O2 concentration (Fig. 3i) were detected in the intestinal tract of BdDuox-silenced flies. The BdDuox knockdown markedly promoted the proliferation of intestinal tract bacteria (Fig. 3j), including S. marcescens and P. alcalifaciens (Fig. 3k, m).
To assess changes in the expression profiles of IMD, Relish, and four effector genes encoding AMPs, including one Diptercin gene and three Cecropin genes, qPCR was used. None of the tested genes was differentially expressed between ds-BdTPH-treated flies and ds-GFP-treated controls (Additional file 1: Fig. S8).
Thus, the data indicate that serotonin regulates gut microbiome homeostasis by controlling Duox expression and therefore ROS activity.
Serotonin-mediated Duox expression in response to gut bacteria in B. dorsalis
Our results indicate that the peripheral serotonin is a key factor maintaining B. dorsalis gut microbiome homeostasis. Next, we assessed whether gut bacteria affect 5-HT biosynthesis. Axenic flies were generated by antibiotic treatment (Fig. 4a, b). Expression of BdTPH was significantly higher in the intestinal tract of axenic flies than in nonaxenic flies (Fig. 4c). No significant difference of BdTRH transcripts were detected between axenic and nonaxenic flies (Fig. 4d). The mRNA level of BdDuox was lower in axenic flies than in nonaxenic flies (Fig. 4e). We then examined whether the expression of BdTPH is correlated with specific members of the fly gut microbiome. We isolated P. alcalifaciens, S. marcescens, and Klebsiella sp. from B. dorsalis guts and these bacteria have been identified as cultivable gut commensals. Among these bacteria, both P. alcalifaciens and S. marcescens robustly proliferated in response to 5-HT elevation. We next cultured these bacteria and orally fed them to antibiotic-treated flies. Gut BdTPH gene was downregulated by oral introduction of P. alcalifaciens and S. marcescens (Fig. 4f), while BdDuox was upregulated after ingestion of P. alcalifaciens and S. marcescens (Fig. 4g). The gut BdTPH mRNA level of Klebsiella sp. treated flies has no significant difference from that of controls (Additional file 1: Fig. S9). These data suggest that the 5-HT regulated Duox expression may be stimulated by specific bacterium.
Serotonin biosynthesis in response to gut bacteria in B. dorsalis. a, b The efficacy of elimination of gut bacteria confirmed by culturing B. dorsalis gut homogenates on LB agar plates (a) and by performing PCR analysis (b) on gut homogenates using universal 16S rRNA gene primers. c–e Relative expression levels of BdTPH, BdTRH, and BdDuox in the gut of nonaxenic and axenic flies. f, g Gene abundance of BdTPH and BdDuox after the bacteria were introduced into the guts of antibiotic-treated flies. Two-tailed unpaired t-test was performed for c–g. Error bars indicate ± s.e.m., **p < 0.01, *p < 0.05, and ns means no significant difference. All results were repeated in at least two independent experiments, with three biological replicates
Serotonin controls Duox expression and gut microbiome load in A. aegypti
Mosquito-borne diseases constitute a large portion of infectious diseases, causing more than 700,000 deaths annually. Aedes mosquito-transmitted diseases, such as dengue, Zika, yellow fever, and chikungunya, have re-emerged recently and remain a public health threat worldwide [26]. The gut commensal microbiome has complicated roles in arboviral infection and transmission in vector mosquitoes [DNA sample preparation and deep sequencing The extraction of total bacterial DNA from the guts of B. dorsalis or A. aegypti was the same as described above. Three replicates were prepared for 1-mM 5-HTP-treated B. dorsalis and the controls. Each replicate contained 10 flies, and the midgut and hindgut were dissected separately. Five replicates were prepared for dsRNA-treated B. dorsalis, and each replicate contained 10 flies. Three replicates were prepared for dsRNA-treated A. aegypti. Each replicate contained 20 mosquitoes, and the midguts were dissected. Approximately 465 bp of the V3+V4 region of the bacterial 16S rDNA gene was amplified by PCR. The following primers were used: F, CCTACGGGNGGCWGCAG; R, GGACTACHVGGGTATCTAAT. PCRs were performed in triplicate in 50 μL mixtures containing 5 μL of 10 × KOD Buffer, 5 μL of 2.5 mM dNTPs, 1.5 μL of each primer (5 μM), 1 μL of KOD Polymerase, and 100 ng of template DNA. Reactions consisted of one cycle at 95 °C for 2 min, followed by 27 cycles at 98 °C for 10 s, 62 °C for 30 s, and 68 °C for 30 s and a final extension at 68 °C for 10 min. The PCR products were checked using 2% agarose gel electrophoresis, purified using an AxyPrep DNA gel extraction kit (Axygen Biosciences, Union City, CA, USA) according to the manufacturer’s instructions, and quantified using fluorescence quantitation (QuantiFluor -TM, Promega, USA). Purified amplicons were pooled in equimolar concentrations and paired-end sequenced (2 × 250) on an Illumina platform according to standard protocols. Data analysis was performed according to Huang et al. [49]. Raw reads were removed if they contained more than 10% of unknown nucleotides (N) or fewer than 80% of bases with quality (Q-value) > 20. Paired-end clean reads were merged as raw tags using FLASH (v1.2.11) with a minimum overlap of 10 bp and mismatch error rate of 2%. Noisy sequences of raw tags were filtered with the QIIME (V1.9.1) pipeline under specific filtering conditions to obtain high-quality clean tags. Clean tags were searched against the reference database (http:// drive5.com/uchime/uchime_download.html) to perform reference-based chimera checking using the UCHIME algorithm (http://www.drive5.com/usearch/manual/uchime_algo.html). All chimeric tags were removed, and the remaining tags were subjected to further analysis. Tags were clustered into operational taxonomic units (OTUs) of ≥97% similarity using the UPARSE pipeline [50]. The tag sequence with the highest abundance was selected as a representative sequence within each cluster. The representative sequences were classified into organisms with a naive Bayesian model using the RDP classifier (version 2.2) based on the SILVA database (https://www.arb-silva.de/). To obtain axenic flies, 5-day-old adults, a mixed of the same number of males and females, were feeding with 5% sucrose solution containing 50 μg/ml tetracycline, 100 μg/mL penicillin-streptomymin, 150 μg/mL gentamycin, and 150 μg/mL rifampicin for 4 days. Controls were fed 5% sterile sugar solution. Flies were then treated with sterile water without antibiotic for 1 day to allow the antibiotics to be metabolized. The flies were decontaminated in 70% ethanol and rinsed in sterile PBS, and the intestinal tracts were dissected under aseptic conditions. Removal of the microorganisms was confirmed by performing PCR analysis using universal 16S rRNA gene primers. To detect the effect of 5-HTP on B. dorsalis mortality, flies were treated with 1-mM 5-HTP in 5% sterile sugar solution daily. Control flies were fed 5% sterile sugar solution only. Experiments were performed independently three times, with three biological replicates, each replicate containing 20 flies. The survival rate was recorded every 24 h. To determine the effect of gut-isolated bacteria on the B. dorsalis survival rate, S. marcescens and P. alcalifaciens were cultured separately overnight at 37 °C. After centrifugation at 7500 rpm for 10 min, bacterial pellets were washed twice with PBS. Then, the pellets were resuspended in sterile 5% sugar solution, reaching OD 600 = 1.5. To infect the flies, 5-day-old B. dorsalis adults, a mix of the same number of males and females, were continuously fed the resulting solution. Control flies were fed a diet supplemented with sterile 5% sucrose only. Surviving flies were counted daily. Experiments were performed independently twice, with three biological replicates, each replicate containing 20 flies. Total RNA was isolated from the guts of 20 adults using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and then treated with RQ1 DNase I (Promega, Madison, WI, USA) to eliminate genomic DNA. Single-strand cDNA synthesized from total RNA aliquots (1 μg) using a RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA, USA) was used as a template for the PCR. The primers are listed in Additional file 2: Table S1. The PCR was performed using Q5 High-Fidelity DNA Polymerase (New England Biolabs), according to the manufacturer’s instructions. To determine their sizes, amplification products were separated by electrophoresis on 1.0% agarose gels. Purified PCR products were then cloned into a pEASY-Blunt Zero Cloning Vector (TransGen, Bei**g, China), following the manufacturer’s instructions, before being sequenced. To analyze gene expression level in the intestinal tract of B. dorsalis, the guts of 5-day-old adults, a mix of the same number of males and females, were dissected. Experiments were performed independently at least twice, with three biological replicates, each replicate containing 10 flies. To analyze gene expression in A. aegypti, midguts of 5-day-old females were dissected. Experiments were performed independently at least twice, with three biological replicates, each replicate containing 20 mosquitoes. RNA was extracted using TRIzol reagent (as described above). The purity of the extracted RNA was assessed spectrophotometrically by measuring the OD260/280 ratio and an OD260/280 of 1.8–2.0 indicates good quality RNA. RNA integrity was measured via electrophoresis on a formaldehyde agarose gel. The RNA aliquots, 1 μg, were reverse transcribed to cDNA using a PrimeScript™ RT reagent Kit with gDNA Eraser (Takara) according to the manufacturer’s instructions. Biosynthesized cDNA was used as a template in the RT-qPCR, conducted on a Stratagene Mx3000P thermal cycler (Agilent Technologies, Wilmington, DE, USA) with TB Green Premix Ex Taq II (Tli RNase H Plus) (Takara Bio, Otsu, Japan). Thermal cycling conditions were the following: 95 °C for 30 s, 40 cycles at 95 °C for 5 s, and 60 °C for 34 s. Three technical replicates were analyzed for each sample. No-template negative controls were included in each run to detect possible contamination or carryover. A series of gene-specific primers were designed for the RT-qPCR using the software Primer 3 (http://bioinfo.ut.ee/primer3-0.4.0/) (Additional file 2: Table S1). The specificity of RT-qPCR reaction products was established via electrophoresis on 1.0% agarose gels before sequencing. The transcript levels of different genes were quantified using the 2−ΔΔCT method [51]. α-tubulin [52] and RpL32 [41] were used as reference genes for gene expression analysis in B. dorsalis, due to their expression stability. The housekee** AaS7 gene was used as an endogenous control for A. aegypti. The gene expression of each sample was normalized to that of controls (taken as 1). PCR-generated DNA templates were used to biosynthesize dsRNA, which contained T7 promoter sequences at each end. The primers used are listed in Additional file 2: Table S1. The amplicons were purified and verified with DNA sequencing. A MEGAscript T7 transcription kit (Ambion, Austin, TX, USA) was used to produce the specific dsRNA of each gene following the manufacturer’s instructions. As a negative control, green fluorescent protein (GFP) dsRNA was biosynthesized. The quality and size of the dsRNA products were verified by 1% agarose gel electrophoresis, and the dsRNA products were diluted with nuclease-free water to the final concentration of 4 μg/μl. The dsRNA, 2 μg, of the target gene or GFP control was injected into the thoracic hemocoel of 5-day-old B. dorsalis adults using a FemtoJet microinjection system (Eppendorf). Gene silencing experiments in A. aegypti were performed by injecting 1.2 μg of dsRNA into the thorax of cold-anesthetized 5-day-old females. The efficiency of dsRNA-mediated gene silencing was determined by RT-qPCR at 1 to 4 days after injection. Experiments were performed independently three times, with three biological replicates. Insects fed sterile sucrose solution. Ten-day-old B. dorsalis adults mixed with the same number of males and females were treated with 5% sucrose solution containing 50 μg/ml tetracycline, 100 μg/mL penicillin-streptomymin, 150 μg/mL gentamycin, and 150 μg/mL rifampicin for 4 days. The flies were then treated with sterile water without antibiotic for 1 day to allow the antibiotics to be metabolized. The flies were decontaminated in 70% ethanol and rinsed in sterile PBS, and the intestinal tracts were dissected under aseptic conditions. Removal of the microorganisms was confirmed by performing PCR analysis using universal 16S rRNA gene primers (Additional file 2: Table S1) and CFU assay. Dissected guts were used for qRT-PCR. Experiments were performed independently twice, with five biological replicates. Serratia marcescens, P. alcalifaciens, and Klebsiella sp. were isolated from the B. dorsalis gut. They were cultured in Luria–Bertani broth at 37 °C for 16 h. The bacterial culture was then pelleted by centrifugation (3000×g, 5 min), washed twice in sterile PBS, and resuspended in 5% sterile sucrose solution ((optical density = 50). The bacterial suspension was provided to the axenic flies for 12 h. All supplies used in the fly feeding experiment were aseptic. The bacteria-fed flies were killed and the guts isolated. The dissected guts were used in RT-qPCR. The intestinal tracts (midgut and hindgut) of B. dorsalis and the midguts of mosquitoes were dissected in PBS containing the catalase inhibitor 3-amino-1,2,4-triazole (A8056, Sigma). Immediately after dissection, the guts were incubated with 2 μM dihydroethidium (DHE, D7008, Sigma) in PBS at room temperature for 30 min in the dark. The guts were then fixed with 4% paraformaldehyde for 30 min and incubated for an additional 30 min with 5% Triton X-100. Fluorescence images were collected using an EVOS FL Auto microscope (Life Technologies, Carlsbad, CA, USA) at 10× magnification. The production of H2O2 was determined according to [36]. A Hydrogen Peroxide Assay Kit (Beyotime Biotech, Shanghai, China) was used according to the manufacturer’s instructions. In this assay, H2O2 converts Fe2+ to Fe3+, which then complexes with xylenol orange dye to yield a purple product having an absorbance maximum at 560 nm, detectable by a spectrometer. The insect guts were dissected in PBS with 2 mg/ml catalase inhibitor 3-amino-1,2,4-triazole (A8056, Sigma). Ten intestinal tracts were dissected for each biological replicate of B. dorsalis. Forty midguts were dissected for each replicate of A. aegypti. The dissected guts were homogenized in 200 μl of lysis buffer and centrifuged at 12,000×g at 4 °C for 5 min, and the supernatant was collected. Aliquots of 50 μl of supernatant and 100 μl of test solution from the hydrogen peroxide assay kit were incubated at room temperature for 20 min and measured immediately with a spectrometer at the wavelength of 560 nm. The concentration of H2O2 released was calculated according to a hydrogen peroxide standard curve. The measurement was repeated three times. Experiments were performed with three biological replicates. Statistical analyses were performed using Prism 5 (GraphPad Software). Two-tailed t-test was used for unpaired comparisons between two groups of data. For the comparison of three or more sets of data, one-way ANOVA was performed, followed by Tukey’s multiple comparison test. A value of p < 0.05 was considered to be statistically significant. Each experiment had three biological replicates.Survival assay
Gene cloning
Reverse-transcription quantitative PCR analysis
dsRNA-mediated gene silencing
Antibiotic treatment of B. dorsalis and oral ingestion of bacteria
In vivo detection of reactive oxygen species
Measurement of hydrogen peroxide production
Statistical analyses