Abstract
Background
Ethyl acetate extracts from Tetrastigma hemsleyanum (Sanyeqing) (EFT), a member of the Vitaceae plant family, have been shown to exhibit efficacy against a variety of cancers. In this light, our current study seeks to examine the mechanism of efficacy between EFT extracts and human pancreatic cancer PANC-1 cells.
Methods
The chemical components of EFT were analyzed by gas chromatography–mass spectrometry. The cytotoxicity of EFT on PANC-1 cells was measured using an MTT assay. In order to investigate EFT induction of cell cycle arrest, changes in cell-cycle distribution were monitored by flow cytometry. Wound healing and transwell assays were employed to investigate whether migration and invasion of PANC-1 cells were inhibited by EFT. Relative protein expression was detected using Western blot.
Results
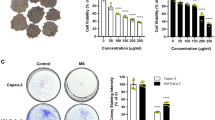
GC-MS analysis of the chemical composition of EFT revealed that the majority of constituents were organic acids and their corresponding esters. EFT exhibits measurable cytotoxicity and inhibition of PANC-1 invasion. Growth inhibition was primarily attributed to downregulation of CDK2 which induces cell cycle arrest in the S-phase. Inhibition of metastasis is achieved through downregulation of mesenchymal-associated genes/activators, including ZEB1, N-cadherin, Vimentin, and Fibronectin. Meanwhile, the expression of E-cadherin was significantly increased by EFT treatment. Furthermore, downregulation of MMP-2 and MMP-9 were observed.
Conclusion
Treatment of PANC-1 with EFT demonstrated measurable cytotoxic effects. Furthermore, EFT evoked S phase arrest while inhibiting the migration and invasion of PANC-1 cells. Additionally, EFT inhibited the epithelial to mesenchymal transition and MMPs expression in PANC-1 cells. This study serves to confirm the strong therapeutic potential of EFT while identifying the mechanisms of action.
Similar content being viewed by others
Background
It is estimated that fatalities arising from pancreatic cancer will be second only to lung cancer by 2030. Overall 5-year survival rates for pancreatic cancer have remained unchanged for decades and patient prognosis remains poor despite efforts to better understand the tumor microenvironment [1,2,3]. Therefore, novel treatment strategies for pancreatic cancer are in dire need. Current chemotherapeutic regimens used in treating pancreatic cancer suffer from debilitating side effects which limit tolerable doses [4]. Furthermore, many therapies exacerbate the mutation of cancers to metastatic and drug resistant phenotypes. Yet, the majority of modern cancer therapeutics are derived from plants [5]. Concerning anti-tumor effects, previous studies have reported that various natural products can demonstrate anticancer efficacy through inhibition of cell growth [5, 6].
Tetrastigma hemsleyanum Diels et. Gilg (Sanyeqing) is a rare, endangered medicinal plant native to China and traditionally used in folk medicine to treat a variety of cancers. Extracts from its root tuber, stems, leaves or whole plant are proven to be effective against various types of cancer [6,7,11, 12]. Characterized by its aggressive biological nature and strong resistance against chemotherapeutic treatment or radiation therapy, the 5-year survival rate of pancreatic cancer has shown little improvement in the past decade at < 5% survival [1, 3, 11,12,13]. Because chemotherapy and radiation therapy produce detrimental side effects, an urgent need for treatment strategies has arisen for pancreatic cancer. Recently, traditional Chinese medicines have garnered increasing attention due to their potential as anti-tumor agents. The majority of traditional Chinese medicines are derived from plant species, which have been used against cancer for centuries [5, 14, 15]. Yet, mechanisms underlying their efficacy remain unclear. Current research suggests that the mechanism of action occurs through cell cycle arrest, induction of apoptosis and inhibition of migration [8, 15,16,17]. Previous studies have shown that an ethyl acetate fraction of Tetrastigma hemsleyanum (EFT) can cause cell cycle arrest and induce apoptosis in human hepatoma HepG2 cells [7]. In this study, the underlying antitumor mechanisms were profiled in PANC-1 human pancreatic cancer cells treated with EFT.
Cell cycle control describes the regulatory process of cell growth that is dictated by coordinated interactions of various cyclins and their respective cyclin-dependent kinases (CDKs) [18, 19]. Cyclins and CDKs together form various checkpoints that can either usher or inhibit progression of a cell through the cell cycle. If the key steps at each regulatory phase of the cell cycle are completed, cyclin/CDK interactions allow progression into the next phase of the cycle [18,19,20]. However, prolonged arrest at a particular checkpoint can occur due to genetic instability or mutation; both key contributors to cancer development [20,21,22]. Not surprisingly, a variety of anticancer agents arrest the cell cycle at particular checkpoints to induce apoptosis in cancer cells [16, 21]. As seen from Fig. 3, S phase arrest was induced by EFT in a dose-dependent manner through downregulation of CDK2. This result indicates that the mechanism of EFT cancer therapy is, in part, due to the downregulation of CDK2 that induces cell cycle arrest and subsequent apoptosis. Yet, the mechanism of EFT is not solely limited to apoptosis. EFT has also shown potential to prevent migration and invasion of PANC-1 cells in a dose-dependent manner in Fig. 4. Since pancreatic cancer is characterized by excessive migration and invasion, methods to specifically inhibit or reverse these malignant features are crucial in both basic and preclinical research [22,23,24]. Largely, EFT’s anti-metastatic properties can largely be related to the epithelial to mesenchymal transition (EMT) and matrix metalloproteinases (MMPs).
EMT is a biological process where cells lose epithelial characteristics and obtain a mesenchymal phenotype, which improves cell motility by decreasing cell-cell adhesion [24]. Many studies point to the activation EMT as the critical mechanism for the acquisition of malignant phenotypes in epithelial cancer cells [24,25,26,27]. EMT is based on the down-regulation of epithelial markers such as E-cadherin while mesenchymal markers, such as ZEB1, Vimentin, N-cadherin and Fibronectin, are up-regulated [28]. Therefore, EMT or mesenchymal properties are an ideal target for cancer therapy.
MMPs are a family of zinc-dependent endopeptidases essential for extracellular matrix degradation [29]. Among all MMPs, MMP-2 and MMP-9 are key to basement membrane type IV collagen degradation during cancer progression. Particularly MMP-2 and MMP-9 are well known for promoting tumor migration and invasion [30, 31]. Aside from direct regulation of basement membrane degradation, cross talk between MMPs and EMT can regulate the invasion or migration of cancer cells. It has been demonstrated that overexpression of MMP-2 or MMP-9 led to induction of EMT in breast cancer [32]. Yet, a variety of EMT processes strengthen MMP expression in return, suggesting an intricate positive feedback loop between MMPs and EMT that synergistically contributes to migration and invasion in malignant tumors.
Therefore, strategies inhibiting both EMT and MMPs are an efficient approach to cancer therapy. As seen from Fig. 5, downregulation of mesenchymal markers such as ZEB1, Vimentin, N-cadherin and Fibronectin and upregulation of epithelial markers such as E-cadherin demonstrate the reversal of the metastatic EMT phenotype induced by EFT. Furthermore, downregulation of MMP-2 and MMP-9 were also identified after EFT treatment. These results suggest that EFT interrupts the positive feedback loop between EMT and MMPs by downregulating key cytokines known for EMT while also downregulating MMPs. These results further contribute to the anti-invasion properties found of EFT against PANC-1 human pancreatic cancer cells.
In summary, pancreatic cancer remains one of the most lethal cancers with 5% of 5-year survival rate. Its poor prognosis is mainly attributed to the nature of the cancer which is both drug resistant and highly metastatic. For treatment of pancreatic cancer, EFT has been identified as a potent therapeutic. As seen from Table 1, GC - MS analysis has revealed that the chemical constituents of EFT are primarily organic acids and their esters, among which succinic acid (10.56%) [33, 34] and benzoic acid (8.65%) had antioxidant, anti-inflammatory effects and had anti-tumor potential. Fatty acids [35,36,37] including linoleic acid (14.37%) and 9,12,15-all-cis-Octadecatrienoic acid (9.46%) have shown activity in cancer prevention and treatment. In recent years, a number of studies have shown that palmitic acid has become a promising antineoplastic agent with demonstrated effects on various malignancies including stomach cancer, liver cancer, cervical cancer, breast cancer and colorectal cancer [38,39,40,41,42,43]. The anti-tumor effects include inducing apoptosis of tumor cells, inhibiting proliferation of tumor cells, inhibiting metastasis and invasion, increasing sensitivity to chemotherapy, and improving immune function. Our findings suggest that EFT contains high levels of palmitic acid (12.02%) and may therefore have the same potential against tumors.
While EFT has shown potent efficacy against PANC-1 cells in vitro, its mechanism of efficacy is poorly understood. Studies performed herein demonstrated that EFT demonstrates significant cytotoxicity against PANC-1 through a cytotoxicity assay. Furthermore, EFT was found to inhibit PANC-1 migration through a wound healing assay. It was determined that EFT inhibits progression of PANC-1 through a collection of key properties. Firstly, EFT halts PANC-1 growth by inducing S-phase cell cycle arrest through downregulation of CDK2. Secondly, EFT tackles the issue of PANC-1 invasion and metastasis through a two-pronged approach. By increasing expression of cell-cell adhesion molecules, EFT substantially reverses the EMT, a key contributor to metastasis. Furthermore, EFT acts to downregulate MMP-2 and MMP-9 which are primarily known for inducing basement membrane degradation. By strengthening the basement membrane and promoting cell-cell adhesion, EFT interrupts the positive feedback loop between EMT and MMPs that are known contributors to the metastasis of PANC-1 cells. Based on the results of this study, the mechanisms by which EFT affects PANC-1 cells are summarized in Fig. 6.
Conclusion
This study confirmed the anti-cancer properties of EFT on human pancreatic cancer PANC-1 cells. EFT exhibits efficacy through multiple avenues; such as inhibition of cell growth, inhibition cell invasion, cell cycle arrest, inhibition of mesenchymal properties and MMPs. In conclusion, these results affirm that Tetrastigma hemsleyanum serves as a valuable therapeutic for the treatment of pancreatic cancer.
Data availability
The data will be accessible by contacting the corresponding author of this study.
References
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–21.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30.
Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144(6):1252–61.
Saung M, T. L Zheng 39 Current standards of Chemotherapy for Pancreatic Cancer. Clin Ther 11 2125–34.
Mbaveng AT, Kuete V, Efferth T. Potential of Central, Eastern and Western Africa Medicinal plants for Cancer Therapy: spotlight on resistant cells and molecular targets. Front Pharmacol. 2017;8:343.
Zhu B, Qian C, Zhou F, Guo J, Chen N, Gao C, ** B, Ding Z. Antipyretic and antitumor effects of a purified polysaccharide from aerial parts of Tetrastigma Hemsleyanum. J Ethnopharmacol. 2020;253:112663.
Peng X, Zhuang DD, Guo QS. Induction of S phase arrest and apoptosis by ethyl acetate extract from Tetrastigma hemsleyanum in human hepatoma HepG2 cells. Tumour Biol. 2015;36(4):2541–50.
**ong Y, Wu X, Rao L. Tetrastigma Hemsleyanum (Sanyeqing) root tuber extracts induces apoptosis in human cervical carcinoma HeLa cells. J Ethnopharmacol. 2015;165:46–53.
Feng Z, Hao W, Lin X, Fan D, Zhou J. Antitumor activity of total flavonoids from Tetrastigma Hemsleyanum Diels et Gilg is associated with the inhibition of regulatory T cells in mice. Onco Targets Ther. 2014;7:947–56.
Lin Z, Chen L, Qiu Q, Guo S. Isolation and identification of antiproliferative compounds from the roots of Tetrastigma hemsleyanum against MDA-MB-435S cell lines. Pak J Pharm Sci. 2016;29(4):1171–5.
Klatte DCF, Wallace MB, Löhr M, Bruno MJ, van Leerdam ME. Hereditary pancreatic cancer. Best Pract Res Clin Gastroenterol. 2022;58–9:101783.
Stoffel EM, Brand RE, Goggins M. Pancreatic Cancer: changing Epidemiology and New approaches to Risk Assessment, early detection, and Prevention. Gastroenterology. 2023;164(5):752–65.
Kunk PR, Bauer TW, Slingluff CL, Rahma OE. From bench to bedside a comprehensive review of pancreatic cancer immunotherapy. J Immunother Cancer. 2016;4:14.
Graham JG, Quinn ML, Fabricant DS, Farnsworth NR. Plants used against cancer - an extension of the work of Jonathan Hartwell. J Ethnopharmacol. 2000;73(3):347–77.
Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. J Clin Oncol. 2009;27(16):2712–25.
Xu B, Shen W, Liu X, Zhang T, Ren J, Fan Y, Xu J. Oridonin inhibits BxPC-3 cell growth through cell apoptosis. Acta Biochim Biophys Sin (Shanghai). 2015;47(3):164–73.
Díaz Osterman CJ, Gonda A, Stiff T, Sigaran U, Valenzuela MM, Ferguson Bennit HR, Moyron RB, Khan S, Wall NR. Curcumin induces pancreatic Adenocarcinoma Cell Death Via reduction of the inhibitors of apoptosis. Pancreas. 2016;45(1):101–9.
Knudsen ES, Kumarasamy V, Nambiar R, Pearson JD, Vail P, Rosenheck H, Wang J, Eng K, Bremner R, Schramek D, Rubin SM, Welm AL, Witkiewicz AK. CDK/cyclin dependencies define extreme cancer cell-cycle heterogeneity and collateral vulnerabilities. Cell Rep. 2022;38(9):110448.
Zhao W, Zhang L, Zhang Y, Jiang Z, Lu H, **e Y, Han W, Zhao W, He J, Shi Z, Yang H, Chen J, Chen S, Li Z, Mao J, Zhou L, Gao X, Li W, Tan G, Zhang B, Wang Z. The CDK inhibitor AT7519 inhibits human glioblastoma cell growth by inducing apoptosis, pyroptosis and cell cycle arrest. Cell Death Dis. 2023;14(1):11.
Bendris N, Lemmers B, Blanchard JM. Cell cycle, cytoskeleton dynamics and beyond: the many functions of cyclins and CDK inhibitors. Cell Cycle. 2015;14(12):1786–98.
Paterna A, Gomes SE, Borralho PM, Mulhovo S, Rodrigues CM, Ferreira MU. Vobasinyl-iboga alkaloids from Tabernaemontana Elegans: cell cycle arrest and apoptosis-inducing activity in HCT116 Colon cancer cells. J Nat Prod. 2016;79(10):2624–34.
Kleeff J, Beckhove P, Esposito I, Herzig S, Huber PE, Löhr JM, Friess H. Pancreatic cancer microenvironment. Int J Cancer. 2007;121(4):699–705.
Razidlo GL, Magnine C, Sletten AC, Hurley RM, Almada LL, Fernandez-Zapico ME, Ji B, McNiven MA. Targeting pancreatic Cancer metastasis by inhibition of Vav1, a driver of Tumor Cell Invasion. Cancer Res. 2015;75(14):2907–15.
Beuran M, Negoi I, Paun S, Ion AD, Bleotu C, Negoi RI, Hostiuc S. The epithelial to mesenchymal transition in pancreatic cancer: a systematic review. Pancreatology. 2015;15(3):217–25.
Wang F, Wang Q, Zhou ZW, Yu SN, Pan ST, He ZX, Zhang X, Wang D, Yang YX, Yang T, Sun T, Li M, Qiu JX, Zhou SF. Plumbagin induces cell cycle arrest and autophagy and suppresses epithelial to mesenchymal transition involving PI3K/Akt/mTOR-mediated pathway in human pancreatic cancer cells. Drug Des Devel Ther. 2015;9:537–60.
Sun XD, Liu XE, Huang DS. Curcumin reverses the epithelial-mesenchymal transition of pancreatic cancer cells by inhibiting the hedgehog signaling pathway. Oncol Rep. 2013;29(6):2401–7.
Qin G, Xu F, Qin T, Zheng Q, Shi D, **a W, Tian Y, Tang Y, Wang J, **ao X, Deng W, Wang S. Palbociclib inhibits epithelial-mesenchymal transition and metastasis in breast cancer via c-Jun/COX-2 signaling pathway. Oncotarget. 2015;6(39):41794–808.
Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119(6):1429–37.
Itoh Y. Membrane-type matrix metalloproteinases: their functions and regulations. Matrix Biol. 2015;44–46:207–23.
Pan F, Ma S, Cao W, Liu H, Chen F, Chen X, Shi R. SDF-1α upregulation of MMP-2 is mediated by p38 MAPK signaling in pancreatic cancer cell lines. Mol Biol Rep. 2013;40(7):4139–46.
Roomi MW, Monterrey JC, Kalinovsky T, Rath M, Niedzwiecki A. In vitro modulation of MMP-2 and MMP-9 in human cervical and ovarian cancer cell lines by cytokines, inducers and inhibitors. Oncol Rep. 2010;23(3):605–14.
Jiang Q, Pan Y, Cheng Y, Li H, Liu D, Li H. Lunasin suppresses the migration and invasion of breast cancer cells by inhibiting matrix metalloproteinase-2/-9 via the FAK/Akt/ERK and NF-κB signaling pathways. Oncol Rep. 2016;36(1):253–62.
Jiang S, Yan W. Succinate in the cancer-immune cycle. Cancer Lett. 2017;390:45–7.
Senthil Kumar KJ, Gokila Vani M, Chen CY, Hsiao WW, Li J, Lin ZX, Chu FH, Yen GC, Wang SY. A mechanistic and empirical review of antcins, a new class of phytosterols of formosan fungi origin. J Food Drug Anal. 2020;28(1):38–59.
Liao P, Wang W, Wang W, Kryczek I, Li X, Bian Y, Sell A, Wei S, Grove S, Johnson JK, Kennedy PD, Gijón M, Shah YM, Zou W. CD8 T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell. 2022;40(4):365–78. e6.
Ma Y, Temkin SM, Hawkridge AM, Guo C, Wang W, Wang XY, Fang X. Fatty acid oxidation: an emerging facet of metabolic transformation in cancer. Cancer Lett. 2018;435:92–100.
Westheim AJF, Stoffels LM, Dubois LJ, van Bergenhenegouwen J, van Helvoort A, Langen RCJ, Shiri-Sverdlov R, Theys J. Fatty acids as a Tool to boost Cancer Immunotherapy Efficacy. Front Nutr. 2022;9:868436.
Siegel I, Liu TL, Yaghoubzadeh E, Keskey TS, Gleicher N. Cytotoxic effects of free fatty acids on ascites tumor cells. J Natl Cancer Inst. 1987;78(2):271–7.
Gärtner S, Krüger J, Aghdassi AA, Steveling A, Simon P, Lerch MM, Mayerle J. Nutrition in Pancreatic Cancer: a review. Gastrointest Tumors. 2016;2(4):195–202.
Riediger ND, Othman RA, Suh M, Moghadasian MH. A systemic review of the roles of n-3 fatty acids in health and disease. J Am Diet Assoc. 2009;109(4):668–79.
Tu QQ, Zheng RY, Li J, Hu L, Chang YX, Li L, Li MH, Wang RY, Huang DD, Wu MC, Hu HP, Chen L, Wang HY. Palmitic acid induces autophagy in hepatocytes via JNK2 activation. Acta Pharmacol Sin. 2014;35(4):504–12.
Kuang H, Sun X, Liu Y, Tang M, Wei Y, Shi Y, Li R, **ao G, Kang J, Wang F, Peng J, Xu H, Zhou F. Palmitic acid-induced ferroptosis via CD36 activates ER stress to break calcium-iron balance in colon cancer cells. FEBS J. 2023;290(14):3664–87.
Wang X, Zhang C, Bao N. Molecular mechanism of palmitic acid and its derivatives in tumor progression. Front Oncol. 2023;13:1224125.
Acknowledgements
We appreciate the great technical support from the Medical Research Center, Academy of Chinese Medical Sciences, Zhejiang Chinese Medical University. We acknowledge the financial and technical supports of the State Administration of Traditional Chinese Medicine of the People’s Republic of China and the Science and Technology Department of Zhejiang Province.
Funding
This research was financially supported by the Specialized Project for Scientific Research of TCM Industry [No. 201407002]; the Science and Technology Program of Zhejiang Province [No. 2016C37069].
Author information
Authors and Affiliations
Contributions
SYF, QHY, ZCC, and ZT carried out GC-MS analysis of the chemical composition of EFT, participated in the cytotoxicity and cell cycle analysis, and drafted the manuscript. SYF and QHY carried out the cell migration and invasion and Western blot analysis. SYF participated in the cell cycle analysis. ZT participated in the design of the study and performed the statistical analysis. XJ and ZT participated in the grammar mistakes correcting and statistical analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Study protocols of plant materials comply with the IUCN Policy Statement on Research Involving Species at Risk of Extinction and the Convention on the Trade in Endangered Species of Wild Fauna and Flora.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sun, Y., Qin, H., Zhang, C. et al. Tetrastigma hemsleyanum (Sanyeqing) root extracts evoke S phase arrest while inhibiting the migration and invasion of human pancreatic cancer PANC-1 cells. BMC Complement Med Ther 24, 133 (2024). https://doi.org/10.1186/s12906-024-04425-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-024-04425-1