Abstract
Purpose
The new grading system for invasive nonmucinous lung adenocarcinoma (LUAD) in the 2021 World Health Organization Classification of Thoracic Tumors was based on a combination of histologically predominant subtypes and high-grade components. In this study, a model for the pretreatment prediction of grade 3 tumors was established according to new grading standards.
Methods
We retrospectively collected 399 cases of clinical stage I (cStage-I) LUAD surgically treated in Tian** Chest Hospital from 2015 to 2018 as the training cohort. Besides, the validation cohort consists of 216 patients who were collected from 2019 to 2020. These patients were also diagnosed with clinical cStage-I LUAD and underwent surgical treatment at Tian** Chest Hospital. Univariable and multivariable logistic regression analyses were used to select independent risk factors for grade 3 adenocarcinomas in the training cohort. The nomogram prediction model of grade 3 tumors was established by R software.
Results
In the training cohort, there were 155 grade 3 tumors (38.85%), the recurrence-free survival of which in the lobectomy subgroup was better than that in the sublobectomy subgroup (P = 0.034). After univariable and multivariable analysis, four predictors including consolidation-to-tumor ratio, CEA level, lobulation, and smoking history were incorporated into the model. A nomogram was established and internally validated by bootstrap**. The Hosmer–Lemeshow test result was χ2 = 7.052 (P = 0.531). The C-index and area under the receiver operating characteristic curve were 0.708 (95% CI: 0.6563–0.7586) for the training cohort and 0.713 (95% CI: 0.6426–0.7839) for the external validation cohort.
Conclusions
The nomogram prediction model of grade 3 LUAD was well fitted and can be used to assist in surgical or adjuvant treatment decision-making.
Similar content being viewed by others
Purpose
Although the existing classification of invasive nonmucinous lung adenocarcinoma (LUAD) is closely related to prognosis, the formal grading system was not conclusive until a new grading system was proposed by the International Association for the Study of Lung Cancer (IASLC) pathology committee and finally included in the 2021 World Health Organization (WHO) Classification of Thoracic Tumors [ Pathological assessments for the training and validation sets are presented in Table 1. According to the IASLC grading system, there were 180 cases (45.1%) of grade 1, 64 cases (16.1%) of grade 2, and 155 cases (38.8%) of grade 3 in the training set. In addition, there were 101 cases (46.8%) of grade 1, 46 cases (21.3%) of grade 2, and 69 cases (31.9%) of grade 3 in the validation set. The baseline features of the training set and validation set are shown in Supplementary Table 1.
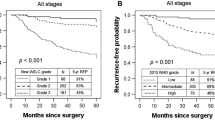
In the training set, a total of 127 patients (81.9%) underwent lobectomy, and 28 (18.1%) underwent sublobectomy in the grade 3 group. Additionally, a total of 194 patients (79.5%) underwent lobectomy, and 50 (20.5%) underwent sublobectomy in the non-grade 3 group. The median follow-up time of the 399 patients was 45.7 months (range: 1.5, 80.0). Significantly worse 5-year RFS rates were observed in the grade 3 group compared to the non-grade 3 group (79.3% vs. 85.9%, P = 0.038, Fig. 2a). Subgroup survival analysis indicated a significantly better RFS rate in grade 3 patients who underwent lobectomy compared to those who underwent sublobectomy (5-year RFS rate 82.0% vs. 67.4%, P = 0.034, Fig. 2b). Conversely, there was no significant difference in RFS between the lobectomy group and the sublobectomy group in the non-grade 3 group (5-year RFS rate 86.8% vs. 82.1%, P = 0.177, Fig. 2c). Furthermore, no significant differences were observed in 5-year OS between grade 3 and non-grade 3 groups (P = 0.45, Fig. 2d). Similarly, no significant difference in 5-year OS was observed between the lobectomy group and the sublobectomy group for both grade 3 and non-grade 3 groups (all P > 0.05), as depicted in Fig. 2e and f. Univariable analysis showed that CTR, lobulation, CEA level, smoking history, and Tdmax were associated with grade 3 tumors (P < 0.05, see Table 2). All the above variables were included in the multivariate logistic regression, and the results showed that CTR, CEA level, lobulation and smoking history were independent risk factors for grade 3 tumors (P < 0.05, see Table 3).
A predictive model was constructed based on the four independent risk factors and visualized with a nomogram (Fig. 3a). Internal validation was performed by bootstrap**, and external validation was completed in an independent patient cohort (Validation set mentioned in the method section) to verify the results. The model had good discrimination (AUC = 0.708, 95% CI: 0.6563–0.7586, Fig. 3b), and calibration curves showed that the predicted probability of the model was very close to the actual probability (Fig. 3c). The Hosmer–Lemeshow test result was χ2 = 7.052 (P = 0.531), indicating that the model underwent a proper calibration. DCA showed that the nomogram prediction model obtained a better net clinical benefit from the intervention decision than the baseline model when the risk was 0.1–0.78 (Fig. 3d). The model discrimination was good in the external validation cohort, showing proper predictive ability (AUC = 0.713, 95% CI: 0.6426–0.7839, Fig. 4a). However, the calibration curve by the bootstrap method showed moderate consistency (Fig. 4b). Development and internal validation of prediction model using training cohort. a The nomogram prediction model for grade 3 tumors in clinical stage I LUAD. b ROC curve for the nomogram (AUC = 0.708). c The calibration curve of the nomogram. d The DCA curve of the nomogram. AUC: area under the receiver operating characteristic curve; CTR: consolidation-to-tumor ratio; Tdmax: maximum tumor diameter; Positive CEA levels represent greater than 4.7 ng/ml and negative levels represent less than 4.7 ng/ml Recently, the IASLC Pathology Committee integrated high-grade subtypes and predominant pathological subtypes to develop a three-tiered grading system for invasive nonmucinous LUAD [26]. Nitadori et al. showed that LUAD patients with greater than or equal to 5% of the micropapillary patterns treated by sublobectomy had a higher risk of recurrence than similar patients treated by lobectomy, suggesting that sublobectomy may not be appropriate for LUAD patients containing any micropapillary components [15]. Song et al. pointed out that lobectomy was recommended for invasive LUAD with pN0 and ≤ 1 cm, and wedge resection can obtain similar oncological effects only for lepidic or acinar predominant tumors [26]. In addition, the survival analysis results in this study suggested that the RFS of the lobectomy group was better than that of the sublobectomy group in grade 3 adenocarcinomas. Therefore, lobectomy is recommended for grade 3 LUAD based on the above results. However, surgical decision-making relies on adequate evaluation of preoperative biopsy specimens or intraoperative frozen sections, whereas treatment options for nonsurgical patients also need to be supported by biopsy pathology results, but these measures have limited ability to evaluate pathological grades [13, 16, 17]. Based on the poor prognosis of grade 3 LUAD, evaluating the pathological grades before treatment, especially grade 3 tumors, can benefit the survival of relevant patients. In this study, the pretreatment prediction model of grade 3 tumors was established and visualized by a nomogram, which can intuitively reflect the probability of grade 3. We proposed a treatment decision diagram based on the grade 3 prediction model, which can provide individual assessment of grade 3 possibilities for each LUAD patient before treatment, thereby optimizing treatment decisions (Fig. 5). The predictors in the model included CTR, lobulation, smoking history, and CEA level, and similar results have been shown in previous studies [27,28,29,30]. Chen et al. defined CTR > 0.5 as radiological invasiveness and used it to construct a radiomics model for predicting micropapillary and solid components, with the sensitivity and specificity reaching 90.00% and 45.21%, respectively [27]. According to the study of Hu et al., there are significant differences in CT morphology between different pathological subtypes, except the acinar and papillary subtypes [28]. Moreover, Yi et al. found that smoking was closely related to micropapillary and solid tumors [29]. The CEA level was proved to be highest in solid-predominant adenocarcinoma and to be independently associated with the presence of solid and micropapillary components, as reported by Li et al. [30]. In addition, the indicators of the nomogram prediction model were readily available in clinical practice, which is suitable for routine application in clinical work. There were some shortcomings in this study, including the following: (i) This study was a single-center retrospective study with a relatively small sample size, and a further prospective study with a large sample size is required in the future; (ii) Temporal validation was selected for external validation in this study, which indicated that multicenter external validation is required in the future to further improve transportability and generalization; and (iii) The clinical and imaging variables included in this study were limited, and in the future, they need to be supplemented and expanded by deep learning, radiomics and other models. Through univariate and multivariate analyses, we found that CTR, lobulation, smoking history and CEA level were independent risk factors for grade 3 tumors and established a pretreatment prediction model for grade 3 tumors. According to the results of survival analysis, the RFS of patients undergoing lobectomy in the grade 3 group was superior to that of patients undergoing sublobectomy. Therefore, in line with previous studies, we suggest that high-risk patients with grade 3 disease screened out by the model should undergo lobectomy, and we have developed a treatment decision diagram that provides convenient conditions for clinical application.Results
Clinicopathologic characteristics
Correlation between surgical extent and prognosis
The risk factors of grade 3 LUAD
Development and validation of the nomogram prediction model
Discussion
Limitation
Conclusion
Availability of data and materials
Due to the privacy of patients participating in the study, the datasets generated or analyzed during the current study are not publicly available, but available from the corresponding author upon reasonable request.
References
Moreira AL, Ocampo PSS, **a Y, et al. A grading system for invasive pulmonary adenocarcinoma: a proposal from the international association for the study of lung cancer pathology committee. J Thorac Oncol. 2020;15:1599–610.
Nicholson AG, Tsao MS, Beasley MB, et al. The 2021 WHO classification of lung tumors: impact of advances since 2015. J Thorac Oncol. 2022;17:362–87.
Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–60.
Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85.
Moreira AL, Joubert P, Downey RJ, Rekhtman N. Cribriform and fused glands are patterns of high-grade pulmonary adenocarcinoma. Hum Pathol. 2014;45:213–20.
Kadota K, Kushida Y, Kagawa S, et al. Cribriform subtype is an independent predictor of recurrence and survival after adjustment for the eighth edition of TNM staging system in patients with resected lung adenocarcinoma. J Thorac Oncol. 2019;14:245–54.
Kadota K, Yeh YC, Sima CS, et al. The cribriform pattern identifies a subset of acinar predominant tumors with poor prognosis in patients with stage I lung adenocarcinoma: a conceptual proposal to classify cribriform predominant tumors as a distinct histologic subtype. Mod Pathol. 2014;27:690–700.
Yang F, Dong Z, Shen Y, et al. Cribriform growth pattern in lung adenocarcinoma: more aggressive and poorer prognosis than acinar growth pattern. Lung Cancer. 2020;147:187–92.
Rokutan-Kurata M, Yoshizawa A, Ueno K, et al. Validation study of the international association for the study of lung cancer histologic grading system of invasive lung adenocarcinoma. J Thorac Oncol. 2021;16:1753–8.
Deng C, Zheng Q, Zhang Y, et al. Validation of the novel international Association for the study of lung cancer grading system for invasive pulmonary adenocarcinoma and association with common driver mutations. J Thorac Oncol. 2021;16:1684–93.
Yoshida C, Yokomise H, Ibuki E, Go T, Haba R, Kadota K. High-grade tumor classified by new system is a prognostic predictor in resected lung adenocarcinoma. Gen Thorac Cardiovasc Surg. 2022;70:455–62.
Wang Y, Yang X, Liu B, et al. Percentage of newly proposed high-grade patterns is associated with prognosis of pathological T1–2N0M0 lung adenocarcinoma. Ann Surg Oncol. 2022;29:4437–47.
Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 3.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2022;20:497–530.
Baig MZ, Razi SS, Muslim Z, Weber JF, Connery CP, Bhora FY. Lobectomy demonstrates superior survival than segmentectomy for high-grade non-small cell lung cancer: the national cancer database analysis. Am Surg. 2021;89:120–8.
Nitadori J, Bograd AJ, Kadota K, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst. 2013;105:1212–20.
Kim TH, Buonocore D, Petre EN, et al. Utility of core biopsy specimen to identify histologic subtype and predict outcome for lung adenocarcinoma. Ann Thorac Surg. 2019;108:392–8.
Yeh YC, Nitadori J, Kadota K, et al. Using frozen section to identify histological patterns in stage I lung adenocarcinoma of ≤3 cm: accuracy and interobserver agreement. Histopathology. 2015;66:922–38.
Sica GYA, Sima CS, Azzoli CG, et al. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol. 2010;34:1155–62.
Qian F, Yang W, Wang R, et al. Prognostic significance and adjuvant chemotherapy survival benefits of a solid or micropapillary pattern in patients with resected stage IB lung adenocarcinoma. J Thorac Cardiovasc Surg. 2018;155:1227–35.
Kagimoto A, Tsutani Y, Kambara T, et al. Utility of newly proposed grading system from international association for the study of lung cancer for invasive lung adenocarcinoma. JTO Clin Res Rep. 2021;2:100126.
Hou L, Wang T, Chen D, et al. Prognostic and predictive value of the newly proposed grading system of invasive pulmonary adenocarcinoma in Chinese patients: a retrospective multicohort study. Mod Pathol. 2022;35:749–56.
Watanabe K, Sakamaki K, Ito H, et al. Impact of the micropapillary component on the timing of recurrence in patients with resected lung adenocarcinoma. Eur J Cardiothorac Surg. 2020;58:1010–8.
Zhao Y, Wang R, Shen X, et al. Minor components of micropapillary and solid subtypes in lung adenocarcinoma are predictors of lymph node metastasis and poor prognosis. Ann Surg Oncol. 2016;23:2099–105.
Tsao MS, Marguet S, Le Teuff G, et al. Subtype classification of lung adenocarcinoma predicts benefit from adjuvant chemotherapy in patients undergoing complete resection. J Clin Oncol. 2015;33:3439–46.
Ma M, She Y, Ren Y, et al. Micropapillary or solid pattern predicts recurrence free survival benefit from adjuvant chemotherapy in patients with stage IB lung adenocarcinoma. J Thorac Dis. 2018;10:5384–93.
Song W, Hou Y, Zhang J, Zhou Q. Comparison of outcomes following lobectomy, segmentectomy, and wedge resection based on pathological subty** in patients with pN0 invasive lung adenocarcinoma ≤1 cm. Cancer Med. 2022;11:4784–95.
Chen LW, Yang SM, Wang HJ, et al. Prediction of micropapillary and solid pattern in lung adenocarcinoma using radiomic values extracted from near-pure histopathological subtypes. Eur Radiol. 2021;31:5127–38.
Hu B, Ren W, Feng Z, et al. Correlation between CT imaging characteristics and pathological diagnosis for subcentimeter pulmonary nodules. Thorac Cancer. 2022;13:1067–75.
Yi JH, Choi PJ, Jeong SS, Bang JH, Jeong JH, Cho JH. Prognostic significance of cigarette smoking in association with histologic subtypes of resected lung adenocarcinoma. Kor J Thorac Cardiovasc Surg. 2019;52:342–52.
Li Z, Wu W, Pan X, et al. Serum tumor markers level and their predictive values for solid and micropapillary components in lung adenocarcinoma. Cancer Med. 2022;11:2855–64.
Acknowledgements
We thank American Journal Experts (AJE) for polishing language of the manuscript.
Funding
Funded by the Natural Funding Project of Tian** Science and Technology Bureau (No. 20JCYBJC01350), Tian** Health Science and Technology Project Key Projects (ZD20023) and Tian** Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-018A).
Author information
Authors and Affiliations
Contributions
Conceptualization, DS and KW; methodology and software, XLiu and YD; data curation, JL; pathological evaluation, HG and MX; manuscript writing, KW; project administration, DS, XLi and MW; funding acquisition, DS. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethics Committee of the Department of Thoracic Surgery, Tian** Chest Hospital. Informed consent was obtained from all subjects and/or their legal guardian(s). Besides, the study was performed in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Supplementary Table 1.
Baseline characteristics of training and validation set.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, K., Liu, X., Ding, Y. et al. A pretreatment prediction model of grade 3 tumors classed by the IASLC grading system in lung adenocarcinoma. BMC Pulm Med 23, 377 (2023). https://doi.org/10.1186/s12890-023-02690-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-023-02690-3