Abstract
Background
Major depressive disorder (MDD) is one of the most common psychiatric disorders and is a great disease burden. However, its underlying pathophysiology and aetiology remain poorly understood. Available evidence suggests that circulating microRNAs (miRNAs) are associated with MDD, but it is still unknown whether miRNAs can predict subsequent incident MDD.
Methods
In this nested case-control study, a total of 104 individuals, who were free of MDD at baseline, from the Women’s Health in Lund Area (WHILA) cohort were included. Among them, 52 individuals developed MDD (cases) during the 5 years follow-up and 52 individuals did not develop MDD (controls). Plasma expression levels of miR-17-5p, miR-134-5p, miR-144-5p, let-7b-5p and let-7c-5p at baseline were assessed using qRT-PCR. Logistic regression was used to estimate the odds of develo** MDD among individuals with different levels of miRNA expression.
Results
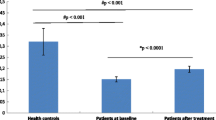
Plasma expression levels of let-7b-5p were significantly lower (p = 0.02) at baseline in cases compared to controls. After adjustment for age and BMI, let-7b-5p was negatively associated with odds for develo** MDD (OR = 0.33, p = 0.03, 95% CI = 0.12–0.91). Moreover, let-7b-5p expression levels showed a trend over time with larger differences between cases and controls for the earlier cases (MDD diagnosis <2 years from baseline) than MDD cases developed later (MDD diagnosis 2–5 years from baseline).
Conclusions
These findings show that lower plasma levels of let-7b-5p are associated with a higher future risk of MDD. Results need to be validated in a large cohort to examine its potential as a peripheral biomarker for MDD.
Similar content being viewed by others
Background
Major depressive disorder (MDD) is a medical condition that includes abnormalities in mood, cognition, appetite, sleep, and psychomotor activity [1]. MDD is one of the most common psychiatric disorders worldwide and is currently projected to become the condition with the greatest disease burden after cardiovascular disease (CVD) by 2030 [2]. As of today, clinical suspicion is still the key for the diagnosis of MDD [3] as the underlying pathophysiology and aetiology of depression remain poorly understood [4].
Previous research concerning the pathophysiology of depression has mainly focused on genetic factors that affect the risk of development of depression. However, few genes were found to affect the risk of development of depression [5]. More recently, the attention has shifted to epigenetics, as it has now been generally accepted that depression is a condition caused not only by genetic factors, but also by the influence of environmental factors on gene expression. Epigenetic mechanisms, including histone modifications, DNA methylation, and a newly recognised group of regulators, the non-coding RNAs (ncRNAs), contribute to the regulation of gene expression [6].
The most common ncRNAs are the microRNAs (miRNAs). MiRNAs are a class of endogenous non-coding single-stranded RNAs of approximately 21–23 nucleotides in length. MiRNAs are transcribed in the nucleus and transported to the cytoplasm where they can inhibit gene expression by either promoting messenger RNA (mRNA) degradation or by inhibiting translation through targeting of the 3′ untranslated region (UTR) of the target mRNA (Bartel [7]). MiRNAs are not only present and active in cells, but they are also observed in a stable, cell-free form. A number of studies have detected miRNAs in peripheral bodily fluids such as the whole blood, plasma, and cerebrospinal fluid (CSF) [8].
Over the past decade, circulating miRNAs have already been identified as potential biomarkers in other diseases such as cancer and diabetes [9,10,11,12]. In MDD, some miRNAs have been evaluated in multiple cohorts. MiR-17-5p was found to be significantly upregulated in patients with MDD compared to healthy controls (HC) [13, 14], whereas the expression of miR-134-5p, miR-144-5p, let-7b-5p and let-7c-5p was significantly downregulated [33] examined the ERK1/2 signaling in the frontal cortex and hippocampus of rats showing vulnerability (learned helplessness (LH)) and rats showing resilience (non-learned helplessness (non-LH)) to the development of stress-induced depression. They determined both activation and expression of ERK1 and ERK2 at the transcriptional and translational level and found both protein and mRNA levels to be significantly decreased in hippocampus and frontal cortex of LH rats. These results suggest the involvement of ERK1/2 signaling in generating vulnerability to depression [33]. Thus, downregulation of let-7b-5p leads to a decrease in ERK1/2 signaling, which in turn is associated with an increased risk of MDD development.
This study is the first nested case-control study to explore potential biomarkers for the risk prediction of MDD development. The results of this study were promising, but the limitations of the study must be kept in mind when evaluating the results. Firstly, the sample size was relatively low. The study design, though, required a smaller minimum sample size than what was used in the study. Secondly, cases and controls were unmatched in the study design. This, however, was corrected via adjustments in age and BMI in the statistical analysis. In addition, family history is a major factor concerning the risk assessment of MDD development [34, 35] The effect of family history, however, could not be assessed as this confounder was not included in the WHILA cohort study [18]. Finally, our research indicates that let-7b-5p has the potential as a circulating predictive biomarker of MDD, but functional studies have to be performed in the future to confirm the potential role of miRNAs in MDD risk prediction.
Conclusions
In summary, the findings of this study show that plasma levels of let-7b-5p have the potential as a biomarker of risk prediction for MDD development. Future studies are needed to validate and confirm the results in different cohorts with larger sample sizes.
Availability of data and materials
The datasets generated during and/or analysed during the current study are not publicly available due to privacy concerns; please contact the corresponding author for more information.
Abbreviations
- MDD:
-
Major depressive disorder
- CVD:
-
Cardiovascular disease
- ncRNA:
-
Non-coding RNA
- miRNA:
-
microRNA
- mRNA:
-
Messenger RNA
- UTR:
-
Untranslated region
- CSF:
-
Cerebrospinal fluid
- HC:
-
Healthy control
- SCZ:
-
Schizophrenia
- BD:
-
Bipolar disorder
- CBT:
-
Cognitive behavioural therapy
- RCT:
-
Randomised control trial
- WHILA:
-
Women’s Health in Lund Area
- CPF:
-
Center for Primary Health Care Research
- ICD:
-
International Classification of Diseases
- Ct:
-
Threshold cycle value
- SD:
-
Standard deviation
- BMI:
-
Body mass index
- TG:
-
Triglycerides
- TC:
-
Total cholesterol
- HDL-C:
-
High-density lipoprotein cholesterol
- LDL-C:
-
Low-density lipoprotein cholesterol
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- CNS:
-
Central nervous system
- BBB:
-
Blood-brain barrier
- TRD:
-
Treatment-resistant depression
- KET:
-
Ketamine treatment
- ECT:
-
Electroconvulsive stimulation
- PBMC:
-
Peripheral blood mononuclear cells
- ERK1/2:
-
Extracellular signal-regulated kinase 1/2
- LH:
-
Learned helplessness
- non-LH:
-
Non-learned helplessness
References
Fava M, Kendler KS. Major depressive disorder. Neuron. 2000;28(2):335–41. https://doi.org/10.1016/S0896-6273(00)00112-4.
Depression and the global economic crisis: is there hope? Lancet. 2012;380(9849):1203. https://doi.org/10.1016/S0140-6736(12)61694-8.
Soleimani L, Lapidus KAB, Iosifescu DV. Diagnosis and treatment of major depressive disorder. Neurol Clin. 2011;29(1):177–93. https://doi.org/10.1016/j.ncl.2010.10.010.
Kennis M, Gerritsen L, van Dalen M, Williams A, Cuijpers P, Bockting C. Prospective biomarkers of major depressive disorder: a systematic review and meta-analysis. Mol Psychiatry. 2020;25(2):321–38. https://doi.org/10.1038/s41380-019-0585-z.
Lohoff FW. Overview of the genetics of major depressive disorder. Curr Psychiatry Rep. 2010;12(6):539–46. https://doi.org/10.1007/s11920-010-0150-6.
Uchida S, Yamagata H, Seki T, Watanabe Y. Epigenetic mechanisms of major depression: targeting neuronal plasticity. Psychiatry Clin Neurosci. 2018;72(4):212–27. https://doi.org/10.1111/pcn.12621.
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97. https://doi.org/10.1016/S0092-8674(04)00045-5.
Maffioletti E, Tardito D, Gennarelli M, Bocchio-Chiavetto L. Micro spies from the brain to the periphery: new clues from studies on microRNAs in neuropsychiatric disorders. Front Cell Neurosci. 2014;8:75. https://doi.org/10.3389/fncel.2014.00075.
Bye A, Røsjø H, Nauman J, Silva GJ, Follestad T, Omland T, et al. Circulating microRNAs predict future fatal myocardial infarction in healthy individuals - the HUNT study. J Mol Cell Cardiol. 2016;97:162–8. https://doi.org/10.1016/j.yjmcc.2016.05.009.
Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating microRNAs as stable blood-based markers for cancer detection. PNAS. 2008;105(30):10513–8. https://doi.org/10.1073/pnas.0804549105.
Salloum-Asfar S, Satheesh NJ, Abdulla SA. Circulating miRNAs, small but promising biomarkers for autism Spectrum disorder. Front Mol Neurosci. 2019;12(253). https://doi.org/10.3389/fnmol.2019.00253.
Wang X, Sundquist J, Zöller B, Memon AA, Palmér K, Sundquist K, et al. Determination of 14 circulating microRNAs in swedes and Iraqis with and without diabetes mellitus type 2. PLoS One. 2014;9(1):e86792. https://doi.org/10.1371/journal.pone.0086792.
Smalheiser NR, Lugli G, Zhang H, Rizavi H, Cook EH, Dwivedi Y. Expression of microRNAs and other small RNAs in prefrontal cortex in schizophrenia, bipolar disorder and depressed subjects. PLoS One. 2014;9(1):e86469. https://doi.org/10.1371/journal.pone.0086469.
Camkurt MA, Acar Ş, Coşkun S, Güneş M, Güneş S, Yılmaz MF, Tamer L. Comparison of plasma MicroRNA levels in drug naive, first episode depressed patients and healthy controls. J Psychiatr Res. 2015;2015(69):67–71. https://doi.org/10.1016/j.jpsychires.2015.07.023.
Hp Z, Xl L, Jj C, Cheng K, Bai SJ, Zheng P, **e P. Circulating microRNA 134 sheds light on the diagnosis of major depressive disorder. Transl. Psychiatry. 2020;10(1):95. https://doi.org/10.1038/s41398-020-0773-2.
Wang X, Sundquist K, Hedelius A, Palmér K, Memon AA, Sundquist J. Circulating microRNA-144-5p is associated with depressive disorders. Clin Epigenetics. 2015;7(1):69. https://doi.org/10.1186/s13148-015-0099-8.
Gururajan A, Naughton ME, Scott KA, O’Connor RM, Moloney G, Clarke G, Dinan TG. MicroRNAs as biomarkers for major depression: a role for let-7b and let-7c. Transl Psychiatry. 2016;6(8):e862. https://doi.org/10.1038/tp.2016.131.
Samsioe G, Lidfeldt J, Nerbrand C, Nilsson P. The Women's health in the Lund area (WHILA) study - an overview. Maturitas. 2010;65(1):37–45. https://doi.org/10.1016/j.maturitas.2009.11.009.
Ding L, Gu H, **ong X, Ao H, Cao J, Lin W, Cui Q. MicroRNAs involved in carcinogenesis, prognosis, therapeutic resistance and applications in human triple-negative breast Cancer. Cells. 2019;8(12):1492. https://doi.org/10.3390/cells8121492.
Vasu S, Kumano K, Darden CM, Rahman I, Lawrence MC, Naziruddin B. MicroRNA signatures as future biomarkers for diagnosis of diabetes states. Cells. 2019;8(12):1533. https://doi.org/10.3390/cells8121533.
Bao MH, Feng X, Zhang YW, Lou XY, Cheng Y, Zhou HH. Let-7 in cardiovascular diseases, heart development and cardiovascular differentiation from stem cells. Int J Mol Sci. 2013;14(11):23086–102. https://doi.org/10.3390/ijms141123086.
Blondal T, Jensby Nielsen S, Baker A, Andreasen D, Mouritzen P, Wrang Teilum M, et al. Assessing sample and miRNA profile quality in serum and plasma or other biofluids. Methods. 2013;59(1):S1–6. https://doi.org/10.1016/j.ymeth.2012.09.015.
Creemers EE, Tijsen AJ, Pinto YM, Rooij E. Circulating MicroRNAs. Circ Res. 2012;110(3):483–95. https://doi.org/10.1161/CIRCRESAHA.111.247452.
Wang KJ, Zhao X, Liu YZ, Zeng QT, Mao XB, Li SN, Chen ZJ. Circulating MiR-19b-3p, MiR-134-5p and MiR-186-5p are promising novel biomarkers for early diagnosis of acute myocardial infarction. Cell Physiol Biochem. 2016;38(3):1015–29. https://doi.org/10.1159/000443053.
Lang MF, Shi Y. Dynamic roles of microRNAs in neurogenesis. Front Neurosci. 2012;6:71. https://doi.org/10.3389/fnins.2012.00071.
Lukiw WJ. Micro-RNA speciation in fetal, adult and Alzheimer's disease hippocampus. Neuroreport. 2007;18(3):297–300. https://doi.org/10.1097/WNR.0b013e3280148e8b.
Cuellar TL, Davis TH, Nelson PT, Loeb GB, Harfe BD, Ullian E, et al. Dicer loss in striatal neurons produces behavioral and neuroanatomical phenotypes in the absence of neurodegeneration. PNAS. 2008;105(14):5614–9. https://doi.org/10.1073/pnas.0801689105.
Bruno DCF, Donatti A, Martin M, Almeida VS, Geraldis JC, Oliveira FS, et al. Circulating nucleic acids in the plasma and serum as potential biomarkers in neurological disorders. Braz J Med Biol Res. 2020;53(10):e9881. https://doi.org/10.1590/1414-431x20209881.
Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403(6772):901–6. https://doi.org/10.1038/35002607.
Lee H, Han S, Kwon CS, Lee D. Biogenesis and regulation of the let-7 miRNAs and their functional implications. Protein Cell. 2016;7(2):100–13. https://doi.org/10.1007/s13238-015-0212-y.
Belzeaux R, Bergon A, Jeanjean V, Loriod B, Formisano-Tréziny C, Verrier L, Ibrahim EC. Responder and nonresponder patients exhibit different peripheral transcriptional signatures during major depressive episode. Transl Psychiatry. 2012;2(11):e185–e185. https://doi.org/10.1038/tp.2012.112.
Ham O, Lee SY, Lee CY, Park JH, Lee J, Seo HH, Hwang KC. Let-7b suppresses apoptosis and autophagy of human mesenchymal stem cells transplanted into ischemia/reperfusion injured heart 7by targeting caspase-3. Stem Cell Res Ther. 2015;6(1):147. https://doi.org/10.1186/s13287-015-0134-x.
Dwivedi Y, Zhang H. Altered ERK1/2 signaling in the brain of learned helpless rats: relevance in vulnerability to develo** stress-induced depression. Neural Plast. 2016:7383724. https://doi.org/10.1155/2016/7383724.
Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157(10):1552–62. https://doi.org/10.1176/appi.ajp.157.10.1552.
Zalar B, Blatnik A, Maver A, Klemenc-Ketiš Z, Peterlin B. Family history as an important factor for stratifying participants in genetic studies of major depression. BJMG. 2018;21(1):5–12. https://doi.org/10.2478/bjmg-2018-0010.
Acknowledgments
We would like to thank science editor, Patrick Reilly, for the critical reading of the manuscript as well as statistician, Karolina Palmér, for her support in the statistical analysis. The authors wish to thank the County Council in Region Skåne for providing financial and administrative support to this study (ALF grant).
Funding
Open access funding provided by Lund University. This work was supported by grants from the Swedish Research Council to Jan Sundquist (2020–01175) and to Kristina Sundquist (2018–02400) as well as ALF funding from Region Skåne awarded to Kristina Sundquist.
Author information
Authors and Affiliations
Contributions
XW, KS, AAM and JS conceived and designed the study; SR and AH performed the experiments and SR performed the statistical analysis; XW, AAM, JS and AH collected the samples and clinical data. SR wrote the first draft and JS, AH, KS, AAM, SR and XW revised the article, and approved the final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was performed in-line with the principles of the Declaration of Helsinki. Sample storage and handling procedures were approved by the Ethical Review Board in Lund. Written informed consent was obtained from all subjects.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Table S1.
Correlation between peripheral blood biomarkers and let-7b-5p expression levels.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Roumans, S., Sundquist, K., Memon, A.A. et al. Association of circulating let-7b-5p with major depressive disorder: a nested case-control study. BMC Psychiatry 21, 616 (2021). https://doi.org/10.1186/s12888-021-03621-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-021-03621-4