Abstract
Background
A complementary feeding (CF) period is necessary for nutritional and developmental reasons. Preterm children encounter more feeding problems than their term counterparts in the CF period. The goal of this study was to develop a nutritional risk screening tool specific to preterm children (the NRSP) in outpatient settings in the CF period, with the expectation of providing a standardised process to determine feeding problems and subsequently offering targeted nutritional advice.
Methods
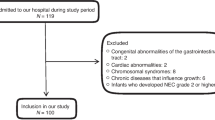
This study was a 2-phase study consisting of the development and evaluation phases. In the development phase, the items of the NRSP were initially developed based on references and the Delphi expert consultation method. Second, 329 preterm individuals with corrected ages from 5 to 36 months were enrolled. The participating preterm children were interviewed with the NRSP and anthropometric measurements, and underwent intellectual developmental tests and biochemistry detection (haemoglobin, red blood cell count, mean corpuscular volume, mean corpuscular haemoglobin, mean corpuscular haemoglobin concentration, serum iron, vitamin D). Third, preterm children’s anthropometric parameters were remeasured 1 month (for infants whose corrected age was 5–11 months) or 3 months (for children whose corrected age was 12–36 months) after the interview. Data in the development phase were analysed via univariate and binary logistic regression analysis sequentially to assign scores for items of the NRSP and to generate the models to predict underweight, stunting, and microcephaly of the NRSP. In the evaluation phase, another 605 preterm individuals were recruited to undergo the interview, anthropometric measurements, intellectual developmental tests, and biochemistry detection as in the development phase. Interrater reliability, test-retest reliability, area under the curve (AUC), accuracy, sensitivity, specificity, the positive/negative predictive value (P/NPV), the positive/negative likelihood ratio (LR+/−), and the correlation coefficient by Spearman’s correlation analysis (rs) were used to assess the reliability and validity of the NRSP. Finally, anthropometric parameters, biochemistry levels, and intellectual development quotients (DQs) from the development and evaluation phases between the high- and low-risk groups classified by the NRSP were compared using a t-test.
Results
The κ coefficients of the interrater and test-retest reliability of the NRSP were all above 0.600, which meant that the reliability of the NRSP was moderate to substantial. The NRSP exhibited relatively higher efficiency in predicting underweight and stunting, with AUCs, accuracies, specificities, and NPVs near to or greater than 0.900, sensitivities above 0.600, PPVs above 0.400, LR + s near to or greater than 10, and rss above 0.400. On the other hand, the NRSP manifested a weaker ability in predicting microcephaly, with most of the values of validity indicators lower than those of underweight and stunting prediction. Z scores of body weight, body length and head circumference, as well as DQs, were all higher in the low-risk groups than in the high-risk groups. There were no significant differences with respect to biochemistry levels between the high- and low-risk groups.
Conclusion
The NRSP shows moderate to substantial reliability and validity in predicting underweight, stunting, and microcephaly. Health care staff should shed light on improving the feeding practices of preterm children with high nutritional risk classified by the NRSP to facilitate their physical growth and intellectual development. More research is expected to promote the NRSP models.
Similar content being viewed by others
Introduction
Clearly, at the corrected age of 4–6 months, preterm infants enter a process when breast milk or formula alone is not sufficient for further nutritional requests such that complementary foods are needed [1, 2]. The appropriate timing of the introduction of complementary foods [3,4,5] and macronutrients and micronutrients provided by complementary foods [6,7,8,9] have positive effects on children’s (including preterm children’s) physical growth and cognitive development. In addition to its nutritional effect, food-related behaviours (such as satiety responsiveness, food fussiness), skills, and attitudes acquired during the complementary feeding (CF) period have long- and short-term health effects [10, 11]. Generally, the CF period is necessary for nutritional and developmental reasons.
However, preterm children encounter more feeding problems than their term counterparts in the CF period, such as improper use of nutritional fortifiers [12, 2.
Models to predict nutritional risks
According to the results of univariate analysis (Additional file 4: Appendix 4, Additional file 5: Appendix 5 and Additional file 6: Appendix 6), binary logistic regression analysis (Additional file 4: Appendix 4, Additional file 5: Appendix 5 and Additional file 6: Appendix 6), references and our group discussions, models to predict nutritional risk were developed as follows.
For preterm infants at the corrected age of 5–7 months
The model to predict underweight included factors of the z-scores for birth weight, the volume of milk intake per day, nutritional fortifier usage, the amount of cereal and animal food intake, food energy density, and recent poor weight gain. The model to predict stunting included factors of the z-scores for birth length, the volume of milk intake per day, nutritional fortifier usage, vitamin D and calcium supplementation, hours spent outdoors per week, and recent poor body length growth. The model to predict microcephaly included factors of the z-scores for birth weight and birth head circumference, the volume of milk intake per day, nutritional fortifier usage, the amount of animal food intake, vitamin D supplementation, hours spent outdoors per week, and recent poor head circumference growth.
For preterm infants at the corrected age of 8–11 months
The model to predict underweight involved factors of the z-scores for birth weight and birth length, the volume of milk intake per day, nutritional fortifier usage, the amount of cereal and animal food intake, food energy density, perceived eating difficulty, and recent poor weight gain. The model to predict stunting involved factors of the z-scores for birth weight and birth length, the volume of milk intake per day, nutritional fortifier usage, the amount of animal food intake, vitamin D, vitamin A and calcium supplementation, hours spent outdoors per week, and recent poor body length growth. The model to predict microcephaly involved factors of the z-scores for birth head circumference, the volume of milk intake per day, iron rich food (red meat, egg and yolk, and animal viscus) intake frequency, the amount of animal food intake, perceived eating difficulty, vitamin D supplementation, hours spent outdoors per week, and recent poor head circumference growth.
For preterm children at the corrected age of 12–36 months
The model to predict underweight covered factors of the z-scores for birth weight and birth length, the volume of milk intake per day, red meat intake frequency, the amount of cereal and animal food intake, food energy density, perceived eating difficulty, and recent poor weight gain. The model to predict stunting covered factors of the z-scores for birth length, the volume of milk intake per day, the amount of animal food intake, perceived eating difficulty, vitamin D and calcium supplementation, hours spent outdoors per week, and recent poor body length growth. The model to predict microcephaly covered factors of the z-scores for birth head circumference, egg and yolk intake frequency, the amount of cereal and animal food intake, perceived eating difficulty, vitamin D supplementation, hours spent outdoors per week, and recent poor head circumference growth.
Reliability
The κ coefficients of the interrater reliability and the test-retest reliability of the NRSP were all above 0.600, which meant that the reliability of the NRSP was moderate to substantial. The data are outlined in Table 3.
Validity
The NRSP exhibited relatively higher efficiency in predicting underweight and stunting, with AUCs, accuracies, specificities, and NPVs near to or greater than 0.900, sensitivities above 0.600, PPVs above 0.400, LR + s near to or greater than 10, and rss above 0.400. On the other hand, the NRSP manifested a weaker ability in predicting microcephaly, with most of the values of validity indicators lower than those of underweight and stunting prediction. Nevertheless, the LR-s of all the predictive models were above 0.1, which suggests less satisfactory results. The data are displayed in Table 3.
We further explored the correlations between the scores of each dimension of the NRSP and malnutrition. We found that the scores of anthropometric assessment were positively correlated with malnutrition in all age groups, except in stunting and microcephaly for preterm infants with a corrected age of 5–7 months. Feeding practices gradually manifested their significantly positive effect as age increased. However, nutrient supplementation did not have a significant correlation with underweight, stunting, or microcephaly. Data are depicted in Table 4.
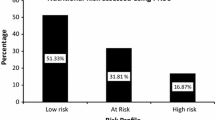
Finally, we compared anthropometric, biochemical, and intellectual developmental indicators between the high and low nutritional risk groups. We found that the z-scores for body weight, body length, and head circumference 1 or 3 months after the first interview were all greater in the low-risk groups versus the high-risk groups. Notwithstanding, there were no significant differences with respect to levels of haemoglobin, RBC count, MCV, MCH, MCHC, serum iron, and vitamin D between the high- and low-risk groups. The full-scale DQs of the high-risk groups were all lower than those of the low-risk groups. Gross motor and social communication DQs were lower in the high-risk group for preterm children with a corrected age of 12–36 months. Fine motor and adaptability DQs were lower in high-risk groups for preterm infants at the corrected age of 5–7, and 8–11 months. Verbal DQ was lower in the high-risk group for preterm infants at the corrected age of 8–11 months. Data are outlined in Table 5.
Discussion
The NRSP was designed for routine clinical use for health care staff when following up with preterm children. The data from this study indicate that the NRSP has acceptable reliability and validity.
The NRSP has moderate to substantial reliability
We used interrater reliability and test-retest reliability to assess the ability of the NRSP in yielding the same nutrition outcome on the same individual. The interrater reliability and the test-retest reliability of the NRSP were all above 0.600, which implies that the reliability of the NRSP is moderate to substantial [34]. It is higher than the reliability of the Paediatric Yorkhill Malnutrition Score (PYMS, κ = 0.53) [35], the Screening Tool for Risk on Nutritional Status and Growth (STRONGkids, κ = 0.483), and the Paediatric Nutrition Screening Tool (PNST, κ = 0.601) [20], but lower than that of the Screening Tool for the Assessment of Malnutrition in Paediatrics (STAMP, κ = 0.882) [21].
The NRSP has moderate to high validity
The AUCs of the NRSP in predicting underweight, stunting, and microcephaly were all above 0.700, which suggests that the effectiveness of the NRSP in malnutrition prediction is relatively high [36]. The AUCs of NRSP were greater than those of the PYMS and the STAMP in predicting wasting (0.717 and 0.657, respectively) and stunting (0.628 and 0.643, respectively) [37]. Sensitivities were all above 0.600, and specificities were all above 0.700 for NRSP, which indicates a moderate to high extent, and they were similar to the sensitivities of the PYMS and STAMP for predicting wasting (0.878 and 0.776, respectively) and stunting (0.724 and 0.759, respectively) [37]. Additionally, the PNST had an approximate sensitivity of 0.88 and a specificity of 0.78, while STRONGkids had a higher sensitivity of 0.94 but a lower specificity of 0.44 [20]. The accuracies of the NRSP in predicting underweight and stunting were near to or above 0.900, which were higher than those of the Subjective Global Nutritional Assessment (SGNA, 67.07%) and the STAMP (45.12%) [38]. The PPVs of the NRSP in predicting underweight and stunting were similar to the SGNA (64.86%) and STAMP (47.06%), while the NPVs of the NRSP were clearly higher than the SGNA (68.89%) and STAMP (47.06%). At the same time, LR + s were higher than the SGNA (2.14) and STAMP (0.93). On the other hand, LR-s were similar to the SGNA (0.52), but lower than the STAMP (1.33) [38]. For a disease with a 10% prevalence, the ideal sensitivity is 90%, specificity is 80%, PPV is 33%, NPV is 98%, LR+ is more than 10, and LR- is less than 0.1 for a diagnostic test [39]. In our study, the total prevalence of malnutrition was 11.43%. Hence, the NRSP has ideal specificity, PPV, NPV, and LR+ in predicting underweight and stunting; however, the NRSP’s potential to predict microcephaly is weaker; moreover, sensitivity and LR- are less favourable for the NRSP. The data revealed that the NRSP classifications were moderately correlated with underweight, stunting and microcephaly, with correlation coefficients varying from 0.355 to 0.558 [40]. This was slightly stronger for the NRSP associated with underweight than stunting or microcephaly, which was the same as reports of the STRONGkids (r = − 0.16 for weight for age, W/A; r = 0.03 for height for age, H/A) [41], SGNA (r = 0.440 for W/A, r = 0.278 for H/A) [42], and PNST (r = 0.66 for W/A, r = 0.19 for H/A) [43]. Further, the NRSP had a stronger correlation with the anthropometry than the STRONGkids, SGNA and PNST. The somewhat weaker validity and correlation of the NRSP with stunting or microcephaly versus underweight might be because body length or head circumference growth are significantly affected by genetic factors, the social and economic environment, cerebral development, and skull thickness compared to mere nutrition factors [44,45,46].
The z-scores for anthropometric parameters and intellectual DQs were significantly higher in the low-risk groups than in the high-risk groups, which indicates that the classification by the NRSP is valid and reasonable. Health care staff should shed light on improving the feeding practices of preterm children with high nutritional risk to facilitate their physical growth and intellectual development. There were no discrepancies with respect to haemoglobin, RBC count, MCV, MCH, MCHC, serum iron, and vitamin D levels between the high- and low-risk groups, which might be because this study was a single-centre investigation, and the participants basically followed the same advice on nutrient supplementation.
Foetal growth status and feeding practices were critical factors for predicting malnutrition
We found that the anthropometric assessment score was positively correlated with malnutrition. The main component of the anthropometric assessment dimension was foetal growth status; therefore, we posited that foetal growth status was of paramount effect on extrauterine growth. A high score for anthropometric assessment indicates worse foetal growth status, which further signals less nutrient storage and a greater probability of disease occurrence, leading to extrauterine growth retardation.
The feeding practice score had a positive correlation with malnutrition only in the corrected 12–36-month age group, and even showed a negative association with malnutrition in the corrected 5–7-month age group. Reasons for the contrary relationship in the early stage might be that in the early stage of preterm birth, because of their low birth weight or length, they are probably regarded as having malnutrition, and the lower their birth weight is, the more likely they are to use nutritional fortifiers. When using nutritional fortifiers, preterm infants obtain lower scores in feeding practices, resulting in a false negative association between the score of feeding practices and malnutrition. As age increases, the effect of foetal growth status might be attenuated, and the positive effect of feeding practices gradually appears.
In the NRSP, some items (such as current diseases and nutrient supplementation, which are recognised in the literature as important factors of nutritional risk prediction) had no significant association with malnutrition in our study, which is contrary to our knowledge. We suspect that this was due to the relatively small sample size from a single centre. We decided to retain these items in the NRSP and anticipate further investigation.
There are limitations in this study. A major limitation was the relatively small sample size. This may have led to the second limitation, which was the moderate reliability and validity of the NRSP. Hence, a large-scale multicentre study should be conducted to broadly promote the NRSP models.
Conclusion
The present study shows that the NRSP has moderate to substantial reliability and validity in predicting underweight, stunting, and microcephaly. Health care staff should shed light on improving the feeding practices of preterm children with high nutritional risk classified by the NRSP to facilitate their physical growth and intellectual development. However, more research is needed to promote the NRSP models.
Availability of data and materials
All data generated or analysed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Abbreviations
- CF:
-
Complementary feeding
- NRSP:
-
Nutritional risk screening tool for preterm children
- AUC:
-
Area under the curve
- PPV:
-
Positive predictive value
- NPV:
-
Negative predictive value
- LR+:
-
Positive likelihood ratio
- LR-:
-
Negative likelihood ratio
- rs :
-
Correlation coefficient by Spearman’s correlation analysis
- DQ:
-
Intellectual development quotient
- RBC:
-
Red blood cell
- MCV:
-
Mean corpuscular volume
- MCH:
-
Mean corpuscular hemoglobin
- MCHC:
-
Mean corpuscular hemoglobin concentration
- WHO:
-
World Health Organization
- SD:
-
Standard deviation
- ANOVA:
-
One-way analysis of variance
- IQR:
-
Interquartile range
- PYMS:
-
Paediatric Yorkhill Malnutrition Score
- STRONGkids:
-
Screening Tool for Risk on Nutritional Status and Growth
- PNST:
-
Pediatric Nutrition Screening Tool
- STAMP:
-
Screening Tool for the Assessment of Malnutrition in Paediatrics
- SGNA:
-
Subjective Global Nutritional Assessment
- W/A:
-
Weight for age
- H/A:
-
Height for age
References
Editorial board of Chinese Journal of Pediatrics, Pediatric health care panel of Chinese Medical Association, neonatology panel of Chinese Medical Association. Feeding suggestions for premature and low birth weight infants after discharge. Chin J Pediatr. 2016;54(1):6–12. https://doi.org/10.3760/cma.j.issn.0578-1310.2016.01.003.
WHO (World Health Organization). 2002. Complementary Feeding. Report of the Global Consultation. Geneva, 10–13 December 2001. Summary of Guiding Principles. http://www.who.int/nutrition/publications/Complementary_Feeding.pdf.
Marriott LD, Foote KD, Bishop JA, Kimber AC, Morgan JB. Weaning preterm infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 2003;88(4):F302–7. https://doi.org/10.1136/fn.88.4.f302 PMID: 12819162; PMCID: PMC1721586.
Spiegler J, Eisemann N, Ehlers S, Orlikowsky T, Kannt O, Herting E, et al. Length and weight of very low birth weight infants in Germany at 2 years of age: does it matter at what age they start complementary food? Eur J Clin Nutr. 2015;69(6):662–7. https://doi.org/10.1038/ejcn.2015.54 PMID: 25872910.
Sun C, Foskey RJ, Allen KJ, Dharmage SC, Koplin JJ, Ponsonby AL, et al. The impact of timing of introduction of solids on infant body mass index. J Pediatr. 2016;179:104–110.e1. https://doi.org/10.1016/j.jpeds.2016.08.064 PMID: 27663213.
Morgan J, Taylor A, Fewtrell M. Meat consumption is positively associated with psychomotor outcome in children up to 24 months of age. J Pediatr Gastroenterol Nutr. 2004;39(5):493–8. https://doi.org/10.1097/00005176-200411000-00009 PMID: 15572888.
Engelmann MD, Sandström B, Michaelsen KF. Meat intake and iron status in late infancy: an intervention study. J Pediatr Gastroenterol Nutr. 1998;26(1):26–33. https://doi.org/10.1097/00005176-199801000-00005 PMID: 9443116.
Neumann CG, Bwibo NO, Murphy SP, Sigman M, Whaley S, Allen LH, et al. Animal source foods improve dietary quality, micronutrient status, growth and cognitive function in Kenyan school children: background, study design and baseline findings. J Nutr. 2003;133(11 Suppl 2):3941S–9S. https://doi.org/10.1093/jn/133.11.3941S PMID: 14672294.
Whaley SE, Sigman M, Neumann C, Bwibo N, Guthrie D, Weiss RE, et al. The impact of dietary intervention on the cognitive development of Kenyan school children. J Nutr. 2003;133(11 Suppl 2):3965S–71S. https://doi.org/10.1093/jn/133.11.3965S PMID: 14672297.
Fewtrell M, Bronsky J, Campoy C, Domellöf M, Embleton N, Fidler-Mis N, et al. Complementary feeding: a position paper by the European Society for Paediatric Gastroenterology, Hepatology, and nutrition (ESPGHAN) committee on nutrition. J Pediatr Gastroenterol Nutr. 2017;64(1):119–32. https://doi.org/10.1097/MPG.0000000000001454 PMID: 28027215.
Boswell N. Complementary feeding methods-a review of the benefits and risks. Int J Environ Res Public Health. 2021;18(13):7165. https://doi.org/10.3390/ijerph18137165 PMID: 34281101.
Lu Y, Mao M, Yang F. Issues regarding breastfeeding in preterm infants. Chin J Pediatr. 2019;57(5):397–9. https://doi.org/10.3760/cma.j.issn.0578-1310.2019.05.018.
**ang Y, Tang QY. Application of breast milk fortifier in breast feeding of very low birth weight infants. J Clin Pediatr. 2019;37(2):158–60. https://doi.org/10.3969/j.issn.1000-3606.2019.02.020.
Braid S, Harvey EM, Bernstein J, Matoba N. Early introduction of complementary foods in preterm infants. J Pediatr Gastroenterol Nutr. 2015;60(6):811–8. https://doi.org/10.1097/MPG.0000000000000695 PMID: 25564809.
Vissers KM, Feskens EJM, van Goudoever JB, Janse AJ. The timing of initiating complementary feeding in preterm infants and its effect on overweight: a systematic review. Ann Nutr Metab. 2018;72(4):307–15. https://doi.org/10.1159/000488732 PMID: 29705810.
Hambidge KM, Sheng X, Mazariegos M, Jiang T, Garces A, Li D, et al. Evaluation of meat as a first complementary food for breastfed infants: impact on iron intake. Nutr Rev. 2011;69(Suppl 1(01)):S57–63. https://doi.org/10.1111/j.1753-4887.2011.00434.x PMID: 22043884; PMCID: PMC3875190.
Sun YL, He XY, Wu CL, Liu K, Ma J. Feeding status of preterm infants during the food conversion period. J Nur Sci. 2019;34(22):33–6. https://doi.org/10.3870/j.issn.1001-4152.2019.22.033.
Medoff-Cooper B, Rankin K, Li Z, Liu L, White-Traut R. Multisensory intervention for preterm infants improves sucking organization. Adv Neonatal Care. 2015;15(2):142–9. https://doi.org/10.1097/ANC.0000000000000166 PMID: 25822519; PMCID: PMC4392385.
Sun YL, He XY, Zeng JY, Wu CL, Liu K, Zeng LY. Preliminary study on responsive feeding of preterm infants during food conversion period. Chin J Child Health Care. 2020;28(1):78–81. https://doi.org/10.11852/zgetbjzz2019-0155.
Carter LE, Shoyele G, Southon S, Farmer A, Persad R, Mazurak VC, et al. Screening for pediatric malnutrition at hospital admission: which screening tool is best? Nutr Clin Pract. 2020;35(5):951–8. https://doi.org/10.1002/ncp.10367 PMID: 31286569; PMCID: PMC7539919.
McCarthy H, Dixon M, Crabtree I, Eaton-Evans MJ, McNulty H. The development and evaluation of the screening tool for the assessment of malnutrition in Paediatrics (STAMP©) for use by healthcare staff. J Hum Nutr Diet. 2012;25(4):311–8. https://doi.org/10.1111/j.1365-277X.2012.01234.x PMID: 22568534.
Hulst JM, Zwart H, Hop WC, Joosten KF. Dutch national survey to test the STRONGkids nutritional risk screening tool in hospitalized children. Clin Nutr. 2010;29(1):106–11. https://doi.org/10.1016/j.clnu.2009.07.006 PMID: 19682776.
Teresa C, Antonella D, de Ville DG. New nutritional and Therapeutical strategies of NEC. Curr Pediatr Rev. 2019;15(2):92–105. https://doi.org/10.2174/1573396315666190313164753 PMID: 30868956.
Lenfestey MW, Neu J. Gastrointestinal development: implications for Management of Preterm and Term Infants. Gastroenterol Clin N Am. 2018;47(4):773–91. https://doi.org/10.1016/j.gtc.2018.07.005 PMID: 30337032.
Voynow JA. "new" bronchopulmonary dysplasia and chronic lung disease. Paediatr Respir Rev. 2017;24:17–8. https://doi.org/10.1016/j.prrv.2017.06.006 PMID: 28697967.
Luu TM, Rehman Mian MO, Nuyt AM. Long-term impact of preterm birth: neurodevelopmental and physical health outcomes. Clin Perinatol. 2017;44(2):305–14. https://doi.org/10.1016/j.clp.2017.01.003 PMID: 28477662.
Chehade H, Simeoni U, Guignard JP, Boubred F. Preterm birth: long term cardiovascular and renal consequences. Curr Pediatr Rev. 2018;14(4):219–26. https://doi.org/10.2174/1573396314666180813121652 PMID: 30101715; PMCID: PMC6416185.
McCarthy EK, Dempsey EM, Kiely ME. Iron supplementation in preterm and low-birth-weight infants: a systematic review of intervention studies. Nutr Rev. 2019;77(12):865–77. https://doi.org/10.1093/nutrit/nuz051 PMID: 31532494; PMCID: PMC6888764.
Burris AD, Burris J, Järvinen KM. Cow's Milk protein allergy in term and preterm infants: clinical manifestations, immunologic pathophysiology, and management strategies. Neoreviews. 2020;21(12):e795–808. https://doi.org/10.1542/neo.21-12-e795 PMID: 33262206.
Abrams SA. Vitamin D in Preterm and Full-Term Infants. Ann Nutr Metab. 2020;76(Suppl 2):6–14. https://doi.org/10.1159/000508421 Epub 2020 Nov 24. PMID: 33232955.
Schwartz E, Zelig R, Parker A, Johnson S. Vitamin a supplementation for the prevention of Bronchopulmonary dysplasia in preterm infants: an update. Nutr Clin Pract. 2017;32(3):346–53. https://doi.org/10.1177/0884533616673613 PMID: 28537511.
Ghomi H, Yadegari F, Soleimani F, Knoll BL, Noroozi M, Mazouri A. The effects of premature infant oral motor intervention (PIOMI) on oral feeding of preterm infants: a randomized clinical trial. Int J Pediatr Otorhinolaryngol. 2019;120:202–9. https://doi.org/10.1016/j.ijporl.2019.02.005 PMID: 30851536.
Spillane A, Belton S, McDermott C, Issartel J, Osborne RH, Elmer S, et al. Development and validity testing of the adolescent health literacy questionnaire (AHLQ): protocol for a mixed methods study within the Irish school setting. BMJ Open. 2020;10(11):e039920. https://doi.org/10.1136/bmjopen-2020-039920 PMID: 33177140; PMCID: PMC7661365.
Chmura-Kraemer H, Periyakoil VS, Noda A. Kappa coefficients in medical research. Stat Med. 2002;21(14):2109–29. https://doi.org/10.1002/sim.1180 PMID: 12111890.
Gerasimidis K, Keane O, Macleod I, Flynn DM, Wright CM. A four-stage evaluation of the Paediatric Yorkhill malnutrition score in a tertiary paediatric hospital and a district general hospital. Br J Nutr. 2010;104(5):751–6. https://doi.org/10.1017/S0007114510001121 PMID: 20398432.
Hai Y, Qin G. Direct estimation of the area under the receiver operating characteristic curve with verification biased data. Stat Med. 2020;39(30):4789–820. https://doi.org/10.1002/sim.8753 PMID: 32944975.
Lee YJ, Yang HR. Comparison of four nutritional screening tools for Korean hospitalized children. Nutr Res Pract. 2019;13(5):410–4. https://doi.org/10.4162/nrp.2019.13.5.410 PMID: 31583060; PMCID: PMC6760980.
Ong SH, Chee WSS, Lapchmanan LM, Ong SN, Lua ZC, Yeo JX. Validation of the subjective global nutrition assessment (SGNA) and screening tool for the assessment of malnutrition in Paediatrics (STAMP) to identify malnutrition in hospitalized Malaysian children. J Trop Pediatr. 2019;65(1):39–45. https://doi.org/10.1093/tropej/fmy009 PMID: 29514329.
Barrett BJ, Fardy JM. Evaluation of diagnostic tests. Methods Mol Biol. 2021;2249:319–33. https://doi.org/10.1007/978-1-0716-1138-8_18 PMID: 33871852.
Schober P, Boer C, Schwarte LA. Correlation coefficients: appropriate use and interpretation. Anesth Analg. 2018;126(5):1763–8. https://doi.org/10.1213/ANE.0000000000002864 PMID: 29481436.
Maciel JRV, Nakano EY, Carvalho KMB, Dutra ES. STRONGkids validation: tool accuracy. J Pediatr. 2020;96(3):371–8. https://doi.org/10.1016/j.jped.2018.12.012 PMID: 31028746.
Pimenta FS, Oliveira CM, Hattori WT, Teixeira KR. Agreement between subjective global nutritional assessment and the nutritional assessment of the World Health Organization. J Pediatr. 2018;94(6):602–8. https://doi.org/10.1016/j.jped.2017.09.007 PMID: 29136495.
White M, Lawson K, Ramsey R, Dennis N, Hutchinson Z, Soh XY, et al. Simple nutrition screening tool for pediatric inpatients. JPEN J Parenter Enteral Nutr. 2016;40(3):392–8. https://doi.org/10.1177/0148607114544321 PMID: 25096546.
Ipsen J, Nowak-Szczepanska N, Gomula A, Aßmann C, Hermanussen M. The association of body height, height variability and inequality. Anthropol Anz. 2016;73(1):1–6. https://doi.org/10.1127/anthranz/2015/0623 PMID: 26754947.
Bach CC, Henriksen TB, Larsen RT, Aagaard K, Matthiesen NB. Head circumference at birth and school performance: a nationwide cohort study of 536,921 children. Pediatr Res. 2020;87(6):1112–8. https://doi.org/10.1038/s41390-019-0683-2 PMID: 31779026.
Catena A, Martínez-Zaldívar C, Diaz-Piedra C, Torres-Espínola FJ, Brandi P, Pérez-García M, et al. On the relationship between head circumference, brain size, prenatal long-chain PUFA/5-methyltetrahydrofolate supplementation and cognitive abilities during childhood. Br J Nutr. 2019;122(s1):S40–8. https://doi.org/10.1017/S0007114516004281 PMID: 28351446.
Acknowledgments
We thank the participants, doctors, and nurses involved in this study.
Funding
The study was funded by a grant from Guangdong Medical Science and Technology Research Fund Project (Grant number A2020472). The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Author information
Authors and Affiliations
Contributions
X.H. and Y.Z. designed the study; X.H., Z.J. and Y.Z. acquired funding; C.W., L.Z., M.Q. and Y.S. collected data; X.H. and Z.J. analysed the data; X.H. and Z.J. wrote the main manuscript. All authors reviewed and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and was approved by the Medical Ethics Committees of Affiliated Foshan Maternity and Child Healthcare Hospital, Southern Medical University (Foshan Maternity and Child Healthcare Hospital), approval number FSFY-MEC-2020-028. Written informed consent forms were voluntarily signed by all participants’ guardians.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest. The funders had no role in the design of the study; in the data collection, analysis, or interpretation; in the writing of the manuscript; or in the decision to publish the results.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Appendix 1
. Nutritional Risk Screening Tool for Preterm Infants at the Corrected Age of 5–7 Months.
Additional file 2: Appendix 2
. Nutritional Risk Screening Tool for Preterm Infants at the Corrected Age of 8–11 Months.
Additional file 3: Appendix 3
. Nutritional Risk Screening Tool for Preterm Children at the Corrected Age of 12–36 Months.
Additional file 4: Appendix 4–1
. Univariate analysis of responses to the screening tool and the z-scores classification of body weight, length and head circumference for preterm infants at the corrected age of 5–7 months [n (%)]. Appendix 4–2. Binary logistic regression analysis of responses to the screening tool and the z-scores classification of body weight, length and head circumference for preterm infants at the corrected age of 5–7 months (P > 0.900 were not shown).
Additional file 5: Appendix 5–1
. Univariate analysis of responses to the screening tool and the z-scores classification of body weight, length and head circumference for preterm infants at the corrected age of 8–11 months [n (%)]. Appendix 5–2. Binary logistic regression analysis of responses to the screening tool and the z-scores classification of body weight, length and head circumference for preterm infants at the corrected age of 8–11 months (P > 0.900 were not shown).
Additional file 6: Appendix 6–1
. Univariate analysis of responses to the screening tool and the z-scores classification of body weight, length and head circumference for preterm children at the corrected age of 12–36 months [n (%)]. Appendix 6–2. Binary logistic regression analysis of responses to the screening tool and the z-scores classification of body weight, length and head circumference for preterm children at the corrected age of 12–36 months (P > 0.900 were not shown).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
He, X., Jiang, Z., Wu, C. et al. Development of a nutritional risk screening tool for preterm children in outpatient settings during a complementary feeding period: a pilot study. BMC Pediatr 22, 702 (2022). https://doi.org/10.1186/s12887-022-03774-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12887-022-03774-5