Abstract
Background
FLI1 is an oncogenic transcription factor that promotes diverse malignancies through mechanisms that are not fully understood. Herein, FLI1 is shown to regulate the expression of Ubiquitin Associated and SH3 Domain Containing A/B (UBASH3A/B) genes. UBASH3B and UBASH3A are found to act as an oncogene and tumor suppressor, respectively, and their combined effect determines erythroleukemia progression downstream of FLI1.
Methods
Promoter analysis combined with luciferase assays and chromatin immunoprecipitation (ChIP) analysis were applied on the UBASH3A/B promoters. RNAseq analysis combined with bioinformatic was used to determine the effect of knocking-down UBASH3A and UBASH3B in leukemic cells. Downstream targets of UBASH3A/B were inhibited in leukemic cells either via lentivirus-shRNAs or small molecule inhibitors. Western blotting and RT-qPCR were used to determine transcription levels, MTT assays to assess proliferation rate, and flow cytometry to examine apoptotic index.
Results
Knockdown of FLI1 in erythroleukemic cells identified the UBASH3A/B genes as potential downstream targets. Herein, we show that FLI1 directly binds to the UBASH3B promoter, leading to its activation and leukemic cell proliferation. In contrast, FLI1 indirectly inhibits UBASH3A transcription via GATA2, thereby antagonizing leukemic growth. These results suggest oncogenic and tumor suppressor roles for UBASH3B and UBASH3A in erythroleukemia, respectively. Mechanistically, we show that UBASH3B indirectly inhibits AP1 (FOS and JUN) expression, and that its loss leads to inhibition of apoptosis and acceleration of proliferation. UBASH3B also positively regulates the SYK gene expression and its inhibition suppresses leukemia progression. High expression of UBASH3B in diverse tumors was associated with worse prognosis. In contrast, UBASH3A knockdown in erythroleukemic cells increased proliferation; and this was associated with a dramatic induction of the HSP70 gene, HSPA1B. Accordingly, knockdown of HSPA1B in erythroleukemia cells significantly accelerated leukemic cell proliferation. Accordingly, overexpression of UBASH3A in different cancers was predominantly associated with good prognosis. These results suggest for the first time that UBASH3A plays a tumor suppressor role in part through activation of HSPA1B.
Conclusions
FLI1 promotes erythroleukemia progression in part by modulating expression of the oncogenic UBASH3B and tumor suppressor UBASH3A.
Similar content being viewed by others
Introduction
UBASH3A (STS-2/TULA/ CLIP4) and UBASH3B (STS-1/TULA-2) belong to the ubiquitin-associated and Src-homology 3 domain-containing (UBASH3) family [1]. While UBASH3B is ubiquitously expressed [2], UBASH3A expression is restricted to lymphoid tissues [3]. UBASH3A is a negative regulator of T-cell activation and function through regulation of ZAP70 and TCR activation [2, 4]. Genetic variants in this gene are associated with several distinct autoimmune diseases [5,6,7,8], including type 1 diabetes, and rheumatoid anthesis, although the underlying mechanism is still unknown. UBASH3A has three structural domains: 1) N-terminal UBA (ubiquitin-associated), 2) SH3 (Src homology 3), and 3) PGM (phosphoglycerate mutase-like/C-terminal histidine phosphatase) domain [8]. The UBA domain can bind to mono-ubiquitin and lysine-63 and methionine-1-linked polyubiquitin chains. UBASH3A has four known ubiquitination sites at lysine residues 15, 202, 309, and 358. Monoubiquitination at Lys 202 causes UBASH3A to adopt a closed conformation, which prevents the binding of the UBA domain to substrates [9]. The SH3 domain interacts with dynamin [10] (which is required for endocytosis) and with CBL [11] (an E3 ubiquitin ligase). The PGM domain mediates self-dimerization [12], which exhibits very weak, possibly acid-dependent, phosphatase activity (despite its structural similarity to the more active PGM domain in UBASH3B) [3, 13].
UBASH3B has similar structural domains to UBASH3A and some overlap** functions. However, UBASH3B suppresses T-cell receptor (TCR) signaling by dephosphorylating ZAP-70 and Syk, two key molecules involved in the amplification of TCR-triggered signals [3, 14, 15]. UBASH3A and UBASH3B knockout mice exhibit no obvious phenotype until the T cell receptor (TCR) is stimulated. Upon stimulation, T cells from UBASH3A / UBASH3B double deficient mice are hyper-proliferative and produce more IL-2 and IFNγ than wild-type T cells [3], underscoring the vital role UBASH3A/B in T cell regulation and autoimmunity. UBASH3B expression is implicated in various cancers through its ability to bind CBL and block its ubiquitination activity [16, 17].
We have previously shown that Protein Kinase C Delta (PKCẟ) downregulation in TPA (4b-12-O-tetradecanoylphobol-13-acetate)-resistant cell lines, can inhibit erythroleukemia when paired with UBASH3B knockdown [18]. Interestingly, TPA and several PKCẟ agonists have previously been reported to activate the transcription factor FLI1 through increased protein phosphorylation [19,20,22, 23]. Human FLI1 oncogene was later implicated in various types of cancers through translocations or overexpression [24,25,26,27,28,29,30,31,32,33,34,35,36,37]. Indeed, Fli-1 transcriptional activation affects several hallmarks of cancer, including proliferation, survival, differentiation, angiogenesis, genomic instability, and immune surveillance [37]. In this study, we show that leukemias expressing high FLI1 produce either higher UBASH3B or lower UBASH3A. We also show that FLI1 controls the expression of these ubiquitin associated ligase genes in erythroleukemic cells. These results suggest that FLI1 promotes erythroleukemia and possibly progression of other cancers in part by balancing oncogenic effect of UBASH3B and tumor suppressor activity of UBASH3A.
Materials and methods
Cells, culture conditions and drug therapy
The human leukemia (HEL 92.1.7, K562) and epithelial-like HEK293T (CRL-3216) cell lines were obtained from ATCC (US) and tested negative for mycoplasma. These cell lines were cultured and maintained in Dulbecco’s Modified Eagle Medium supplemented with HyClone 5% fetal bovine serum (GE Healthcare, US).
For drug treatment, cells were treated with Camptothecin (MedChemExpress, CN), T5224 (APExBIO, CN) and R406 (Beyotime Biotechnology, CN) for indicated times and used for cell proliferation analysis. Generation of K562-fli1 cells was previously described [21]. For FLI1 induction, cells were treated with 5μM of doxycycline (Solarbio, CN).
RNA preparation and RT-qPCR
Total RNA was extracted using Trizol reagent (Thermo Fisher Scientific, US), cDNA was synthesis using the PrimeScript RT Reagent Kit (Takara Bio, CN) and RT-qPCR analysis using the FastStart Universal SYBR Green Master Mix (Roche, CH) on a Step One Plus Real-time PCR system (Applied Biosystems/Thermo Fisher Scientific, US). The expression of the test genes was given as relative to β Actin. Three biological replicates in triplicate (n = 3) were performed for each gene. The primers sequences were listed in the Table 1.
Promoter analysis and luciferase assays
The UBASH3A and UBASH3B promoter regions (see Figs. 2A and 3A) were amplified by PCR, cloned into the luciferase reporter vector pGL3-basic (Promega, US), and used in a luciferase activity assay, as previously described [38]. Briefly, 2.5 μg of the indicated promoter was co-transfected with either MigR1 (2.5 μg) or MigR1-FLI1 (2.5 μg) using a Lipofectamine 2000 kit (Thermo Fisher Scientific) into epithelial HEK-293T cells which seeded onto 6-well plates one day before, according to the manufacturer’s protocol. Renilla luciferase (Promega, US) was used as an internal control for transfection efficiency.
ShRNA and siRNA expression
The construction of shFLI1 cells has been previously described [38]. shUBASH3A, shUBASH3B, and shHSPA1B and scrambled control vectors were generated by inserting the corresponding shRNA sequence containing oligonucleotides and scrambled DNAs into the restriction enzyme sites BcuI within the PLent-GFP expression vector (obtained from Vigene Bioscience, US). The lentivirus particles were generated by co-transfecting shRNA PLent-GFPs (10 µg) with packaging plasmids psPAX2 (5 µg) and pMD2G (10 µg) (Addgene plasmid #12,259 & #12,260) into HEK293T cells, using lipofectamine 2000. The supernatants were collected two days after transfection to transduce HEL cells. The positive cells were then selected via incubation with a medium containing puromycin (5 µg/ml; Solarbio, CN). ShRNA sequences are listed in Table 2.
UBASH3A siRNAs and negative control were purchased from GenePharma (CN). The UBASH3A siRNA was transfected into shUBASH3B cells using Lipofectamine 2000. Two days after transfection, cells were collected, RNA was extracted, and RT-qPCR was used to detect UBASH3A. For proliferation analysis, cells were transfected with siRNA for 48 h and assessed using an MTT assay every day for three days. SiRNA sequences listed in Table 3.
RNAseq analysis and bioinformatics
Total RNA samples isolated from designated cells and appropriate controls were sent for RNAseq at BGI genomics (CN). BGI also performed the data preprocessing, which we used to analyze the gene expression profiles between shRNA-mediate knocked downed genes and the scrambled control group. Differentially expressed genes (DEGs) were determined by the condition (log2FoldChange ≤ -1 or ≥ 1, padj < 0.05) and then analyzed by Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment. Differentially expressed genes (DEGs) for UBASH3B and UBASH3A were shown in Supplementary Tables 1 and 2, respectively. The TCGA data analysis was obtained using GEPIA2 resources (http://gepia2.cancer-pku.cn/).
Western blotting
Total protein from cell lines was extracted using RIPA buffer (Beyotime Institute of Biotechnology, CN) containing 1:100 PMSF (Solarbio, CN). The protein concentration was determined using a BCA kit (Solarbio, CN) according to the manufacturer's protocol. Load equal amounts of protein into the wells of the SDS-PAGE gel and transferred to PVDF membrane. The membrane was blocked using non-fat milk for 1 h at room temperature. Incubate the membrane with primary antibody in blocking buffer overnight at 4°C. After washed by TBST (Beyotime Institute of Biotechnology, CN) at room temperature for three times, the membrane was incubated with Anti-rabbit IgG (H + L) DyLight™ 800 4X PEG Conjugated secondary antibody (5151s, Cell Signaling Technology, US) in blocking buffer at room temperature for 1 h. The following primary antibodies were used: anti-FLI1 (ab133485, Abcam, UK), anti-UBASH3A (15,823–1-AP, Proteintech, DE), anti-UBASH3B (19,563–1-AP, Proteintech, DE), polyclonal rabbit test primary antibodies; anti-GAPDH (G9545, Sigma Aldrich, US). Antibody dilution was conducted according to the manufacturer’s instructions. The Odyssey system (LICOR Biosciences) was used for western blot membrane imaging and analysis.
Apoptosis
Cells were incubated with compounds or vehicle for 24 h, as previously described [4A). In this analysis, 113 DEGs were common between shFLI1, shUBASH3A and shUBASH3B cells (Fig. 5E). In addition to DEGs observed in both UBASH3A and UBASH3B cells, we identified DEGs unique to one of the two ubiquitin associated ligases (Fig. 5E). A comparison between upregulated or downregulated DEGS from UBASH3B and UBASH3A cells is shown in Supplemental Fig. 5A-D. This analysis reveals upregulation of MAP Kinase pathway genes in UBASH3B and downregulation in UBASH3A cells. The common DEGs in the MAP Kinase pathway and expression variation between the shUBASH3A, shUBASH3B and shFLI1 effected genes are shown as a heatmap (Fig. 5F and Supplemental Fig. 4B). These changes may partially account for the suppressive and oncogenic differences between these UBASH3 isoforms in leukemia cells.
Regulation of the MAP Kinase pathway via UBASH3A and UBASH3B. A Compared to scrambled controls, many genes were upregulated or downregulated in shFLI1, shUBASH3A1 and shUBASH3B cells. B, C KEGG pathway enrichment analysis for shUBASH3A (B) and shUBASH3B cells (C). D KEGG pathway enrichment analysis for DEGs commonly affected by both UBASH3A and UBASH3B genes belonging to the MAP Kinase pathway. E Number of common or unique DEGs in shFLI1, shUBASH3A and UBASH3B cells. F Heatmap showing the differentially expressed MAP Kinase genes in shUBASH3A1 and shUBASH3B cells
UBASH3B ablation activates the AP1(FOS-JUN) pathway and blocks expression of SYK to inhibit leukemia proliferation
The MAP Kinase pathway (Fig. 5F) heatmap revealed that FOS and JUN expression was elevated in shUBASH3B knock-down cells (compared to shUBASH3A and scrambled control cells), and this was further confirmed by RT-qPCR (Fig. 6A-C). The AP1 genes were also induced in shFLI1 cells (Supplemental Fig. 4B). Moreover, overexpression of FLI1 in K562 (K562-fli1) cells resulted in downregulation of both FOS and JUN (Supplemental Fig. 6A-C). Since elevated FOS and JUN expression was associated with growth suppression in shUBASH3B cells, we treated shUBASH3B cells with the selective AP1 inhibitor T5224 [40], which significantly accelerated their proliferation (Fig. 6D). Moreover, T5224 significantly inhibited camptothecin (CPT, an anti-FLI1 compound [20, 41, 42])-induced HEL apoptosis in culture (Fig. 6E, F).
AP1 /SYK are regulated by UBASH3B. A-C Expression of UBASH3B (A), JUN (B), and FOS (C) was assessed by RT-qPCR in shUBASH3B cells. D The proliferation of shUBASH3B cells treated with the selective AP1 inhibitor T5224 (10μM) compared to vehicle-treated (DMSO) cells. E HEL cells were treated with 10nM camptothecin (CPT) (a FLI1 inhibitor) in combination with either DMSO or T5224 for 24 h; apoptosis was measured using flow cytometry. F The data is presented using the average from three experiments. G, H The expression of SYK in shFLI1 (G) and shUBASH3B (H) versus control cells, via RT-qPCR. I The proliferation of HEL cells treated with the SYK inhibitor R406 compared to vehicle-treated (DMSO). P < 0.05 (*), P < 0.01 (**), P < 0.001 (***), and P < 0.0001 (****)
RNAseq analyses in shFLI1 identified drastic downregulation in expression of the spleen tyrosine kinase SYK that was also detected in shUBASH3B cells, suggesting regulation of these genes by FLI1 [36]. The SYK gene has been previously linked to leukemia progression [43]. Indeed, RT-qPCR analysis confirmed downregulation of SYK in shFLI1 and shUBASH3B cells (Fig. 6G, H). Treatment of HEL cells with SYK inhibitor R406 [44] significantly suppressed growth in culture (Fig. 6I). These results suggest that UBASH3B may partially exert its oncogenic activity by suppressing AP1 and activating other oncogenic factors.
HSPA1B suppression by UBASH3A accelerates leukemia cell proliferation
Interestingly, expression of both Heat Shock Protein Family A (Hsp70) Member 1A (HSPA1A) and 1B (HSPA1B) increased in shUBASH3A and shUBASH3B cells relative to controls (Fig. 5F). The induction of HSPA1B in shUBASH3A1 and shUBASH3B was confirmed by RT-qPCR (Fig. 7A and B). Likewise, HSPA1B expression was significantly induced in shFLI1 cells (Fig. 7C, D and Supplemental Fig. 4B). Moreover, overexpression of FLI1 in K562 (K562-fli1) cells resulted in downregulation of HSPA1B (Supplemental Fig. 6A and D) suggesting a tumor suppressor role for this gene. To determine whether HSPA1B is involved in UBASH3A/B mediated tumor suppression, HSPA1B was then knocked-down in HEL cells using three shRNA (shHSPA1B-3; Fig. 7E). The proliferation of shHSPA1B-3 cells was significantly higher than in scrambled control cells (Fig. 7F). Thus, HSPA1B may mediate suppressive activity of FLI1. Since UBASH3A is induced in shFLI1, the level of HSPA1B expected to be lower causing cell growth acceleration. In contrast, lower UBASH3B expression in shFLI1 cells caused higher expression of HSPA1B, leading to growth deceleration. In the schematic in Fig. 7G, we propose that the oncogenic activity of FLI1 through UBASH3B activation may be partly mediated through AP1 suppression in erythroleukemic cells. Previously, we showed that UBASH3B upregulation increases PKCẟ degradation, which increased drug resistance and leukemia cell survival [18]. UBASH3B also activates the oncogene SYK to promote leukemia growth. Since HSPA1B is negatively regulated by both UBASH3A and UBASH3B, its tumor suppressor activity is dependent upon balance between the level of these UBASH3 proteins, negatively and positively regulated by FLI1, respectively. The balance between oncogenic and tumor suppressor activity of UBASH3B and UBASH3A, respectively, likely contributes to FLI1-induced leukemia cell proliferation.
Negative regulation of the HSPA1B by UBASH3A controls cell proliferation. A, B Expression of HSPA1B in shUBASH3B (A) and shUBASH3A1 (B) cells, via RT-qPCR. C, D Expression of FLI1 (C) and HSPA1B (D) in shFLI1 cells via RT-qPCR. E lentivirus-mediated downregulation of HSPA1B in HEL cells using the shHSPA1B1-3 expression vector, as determined via RT-qPCR. F The proliferation of shHSPA1B3 and scrambled control cells for the indicated days was assessed using an MTT assay. P < 0.01 (**), P < 0.001 (***). G Model showing the effect of FLI1 on UBASH3A and UBASH3B expression as well as erythroleukemia progression. UBASH3B induction via FLI1 overexpression suppresses PKCẟ and increased cell survival as well as drug resistance. Higher UBASH3B transcription following FLI1 overexpression also causes inhibition of AP1, which would otherwise suppress leukemia progression. In addition, UBASH3B controls the expression of SYK and partially contributes to erythroleukemia progression. Suppression of UBASH3A transcription via FLI1 overexpression increases the expression of leukemia growth suppressor HSPA1B, which blocks proliferation. On the other hand, activation of UBASH3B by FLI1 further decreases HSPA1B expression, causing acceleration of cell proliferation. Dotted lines represent indirect regulation
Correlation between FLI1 and the UBASH3A/B gene expression in other malignancies and prognostic impact
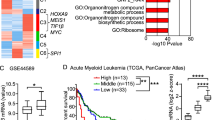
The aforementioned results demonstrated a positive and negative correlation between FLI1 and the UBASH3B and UBASH3A genes in erythroleukemia cell lines, respectively. To examine a broader role of these UBASH genes in cancer, we examined the correlation between FLI1 and UBASH3A or UBASH3B in the TCGA database by GEPIA2. In most tumors, expression analysis revealed a higher level of UBASH3B versus normal samples (Fig. 8A). In Acute Myeloid Leukemia (AML) and whole blood cells, the expression of FLI1 was significantly correlated with the level of UBASH3B (Fig. 8B, C). Higher UBASH3B in AML, Pancreatic Adenocarcinoma, Brain lower grade glioma, Pancreatic adenocarcinoma and Lung squamous cell carcinoma were also correlated with worse prognosis (Fig. 8D, E and Supplementary Fig. 7A-C). These results further support the oncogenic function of UBASH3B in different tumors.
Correlation between FLI1 and UBASH3B expression in various tumors. A Relative expression of UBASH3B in various tumor in comparison to normal cells. Adenoid Cystic Carcinoma (ACC), Bladder Urothelial Carcinoma (BLCA), Breast Invasion Carcinoma (BRCA), Cervical Squamous Cell Carcinoma and Endocervical Adenocarcinoma (CESE), Cholangiocarcinoma (CHOL), Colonadenocarcinoma (COAD), Lymphoid Neoplasm Diffuse Large B-Cell Lymphoma (DLBC), Esophageal Carcinoma (ESCA), Glioblastoma Multiform (GBM), Head and Neck Squamous Cell Carcinoma (HNSC), Kidney Chromophobe (KICH), Kidney Renal Clear Cell Carcinoma (KIRC), Kidney Renal Papillary Cell Carcinoma (KIRP), Acute Myeloid Leukemia (LAML), Brain Lower Grade Glioma (LGG), Liver Hepatocellular Carcinoma (LIHC), Lung Adenocarcinoma (LUAD), Lung Squamous Cell Carcinoma (LUSC), Ovarian Serous Cystadenocarcinoma (OV), Pancreatic Adenocarcinoma (PAAD), Pheochromocytoma and Paraganglioma (PCPG), Prostate Adenocarcinoma (PRAD), Rectum Adenocarcinoma (READ), Sarcoma (SARC), Skin Cutaneous Melanoma (SKCM), Stomach Adenocarcinoma (STAD), Testicular Germ Cell Tumors Thyroid Adenocarcinoma (TGCT), (THCA), Thymoma (THYM), Uterine Corpus Endometrial Carcinoma (UCEC), Uterine Carcinosarcoma (UCS). B, C Relative expression of human FLI1 and UBASH3B in AML (B) and whole blood cells (C). D, E Overall survival rate of high and low UBASH3B expression in AML (D) and PAAD (E) tumors. Abbreviations n(high) and n(low) shows the number of patients in UBASH3B high and low expressing tumors
A positive and negative correlations between FLI1 and UBASH3A were observed in various tumors (Fig. 9A). Interestingly, positive correlation between FLI1 and UBASH3A were seen in AML and whole blood cells (Fig. 9B and C). However, higher expression of UBASH3A had a better prognosis outcome in diffuse large B-cell lymphoma, Breast invasive Carcinoma, Colon adenocarcinoma, Head and neck squamous cell carcinoma, Liver hepatocellular carcinoma and Skin cutaneous melanoma (Fig. 9D and Supplementary Fig. 8A-E). Thymoma was the only tumor in which higher UBASH3A was significantly associated with worse patient outcome (Fig. 9E). These results suggest a tumor specific dependent suppressor function for UBASH3A.
Correlation between FLI1 and UBASH3A expression in various tumors. A Relative expression of UBASH3A in the indicated tumors in comparison to normal cells. B, C Relative expression of human FLI1 and UBASH3A in AML (B) and whole blood cells (C). D, E Overall survival rate of high and low expression of UBASH3A in DLBC (D) and PAAD (E) tumors
Discussion
The ETS oncogene FLI1 is a major driver of tumor initiation and progression of diverse types of malignancies [38]. FLI1 regulated genes have been identified to control various cancer hallmarks including cell proliferation, differentiation, apoptosis, genomic stability, and immunity [37]. The combined effect of these downstream effectors contributes to robust oncogenic activity associated with FLI1 overexpression. Herein, we show that both UBASH3A and UBASH3B are strong downstream targets of FLI1. UBASH3B was found to be a direct target of FLI1, and its activation promotes erythroleukemia growth. In contrast, UBASH3A is indirectly downregulated by FLI1 through GATA2 or possibly other transcription factors and likely acts as an inhibitor of erythroleukemic cell proliferation. RNAseq analysis identified distinct and overlap** downstream pathways for UBASH3A and UBASH3B that likely contribute to their suppressive and oncogenic activity, respectively. This study provides novel insights into the role of these factors in leukemia progression.
In Acute Myeloid Leukemia (AML) induced by the oncogene AML-ETO, UBASH3B inactivates CBL, which is predicted to inhibit the ubiquitination of its downstream effectors responsible for leukemogenesis [16]. Similarly, in triple negative breast cancer, higher expression of UBASH3B promotes dephosphorylation and inactivation of CBL, which in turn loses ability to ubiquitinate and induce degradation of the epidermal growth factor receptor (EGFR), leading to accelerated cancer progression [17]. We also previously identified PKCẟ as one of its downstream targets of UBASH3B [18]. Interaction between UBASH3B and PKCẟ accelerated ubiquitination of this kinase, resulting in leukemia cell survival and drug resistance. Moreover, a positive correlation between FLI1/UBASH3B was observed in several cancer types associated with worse prognosis. These results confirm oncogenic activity of UBASH3B in erythroleukemia and likely other cancers.
In a previous study [45], we reported regulation of FOS and JUN by FLI1 in leukemic cells. Herein, we showed that loss of FLI1 and consequently its downstream target UBASH3B in leukemia cells increased AP1 expression, leading to proliferation suppression and increased apoptosis. While AP1 is shown here to function as a tumor suppressor gene downstream of UBASHB, this transcription factor is also known to function as an oncogene in various cancers [46]. Like TGF signaling, the AP1 function in cancer could go both ways [47]. In our study, AP1(FOS and JUN) expression is negatively regulated during leukemia progression. Indeed, JUNB and JUNA are found critical downstream effectors of the tumor suppressor activity of another ETS gene family SPI1/PU.1, and that reduced expression of JUNB shown to be a common feature of acute myeloid leukemogenesis [48]. Since FLI1 knockdown or overexpressing cells exhibit increased or decreased expression of the AP1 genes, respectively [45], we propose a tumor suppressor role for AP1 in erythroleukemia. In addition to AP1, we identified the activation of the SYK gene by FLI1 through UBASH3B. Dephosphorylation of SYK and SAP70 by UBASH3B, two main factors involved in TCR signaling, was previously reported [3, 14, 15]. However, SYK kinase activation is also implicated in leukemia progression [43]. Thus, SYK activation likely contributed to the oncogenic activity of FLI1 through UBASH3B. The mechanisms by which UBASH3B suppresses AP1 transcription and activates SYK has yet to be determined. However, the interaction between UBASH3B and CBL or downregulation of PKCẟ may modify FOS/JUN and SYK regulation. This notion remains to be investigated in future studies.
Despite critical involvement in autoimmunity, the connection between UBASH3A and cancer has not yet been established. In contrast to UBASH3B, knockdown of FLI1 in erythroleukemia cells upregulates UBASH3A expression, raising the possibility of a tumor suppressor function for this variant. In support of this observation, ablation of UBASH3A in high FLI1 expressing erythroleukemic cells significantly accelerated cell proliferation in culture. Interestingly, UBASH3A expression was both induced and reduced relative to normal cells in various cancers. However, higher expression of UBASH3A was found to be a good prognosis marker for patient survival in most tumors, further supporting its anti-cancer activity. FLI1 indirectly controls the transcription of UBASH3A, likely through GATA2, which may warrant further investigation in future studies.
RNAseq analysis of UBASH3A and UBASH3B knocked-down cells revealed the highest effects on the MAP Kinase pathway. Specifically, expression of HSPA1A and HSPA1B increased in both shUBASH3B and shUBASH3A cells. Knockdown of HSPA1B in leukemia cells accelerated leukemogenesis indicating a role for these genes as negative regulators of leukemic cell growth. Interestingly, higher HSPA1A and HSPA1B expression was previously linked to poor survival in colon cancer. In hepatocellular carcinoma (HCC), expression of HSPA1B increased through Hepatitis B virus-mediated activation of ATF7, which accelerated cell proliferation by inhibiting apoptosis [49]. In contrast to solid tumors, the data presented herein suggest an inhibitory role for HSPA1B in leukemia progression, whose expression depend upon the level of UBASH3A and UBASH3B.
Finally, UBASH3A and UBASH3B knockdown affected similar as well as unique genes, as shown here for AP1, SYK and HSPA1B. Thus, the combined oncogenic and tumor suppressor activities of UBASH3A and UBASH3B and their downstream effectors influence leukemogenesis. Examining other genes regulated by UBASH3A and UBASH3B could further determine their role in leukemogenesis, and uncover additional therapeutic targets.
Conclusions
FLI1 is shown in this study to promote erythroleukemia progression by inhibiting UBASH3A and expression and inducing UBASH3B expression. UBASH3B acts as an oncogene to block the AP1 pathway and activate other genes, whereas UBASH3A transcriptional inactivation by FLI1 suppressed expression of HSPA1B. These results uncover critical roles of UBASH3A and UBASH3B in FLI1-driven leukemias.
Availability of data and materials
The datasets generated and/or analysed during the current study are available in the Sequence Read Archive (SRA) repository, https://www.ncbi.nlm.nih.gov/bioproject/1014802.
Abbreviations
- FLI1:
-
Friend leukemia integration 1
- UBASH3A:
-
Ubiquitin Associated and SH3 Domain Containing A
- UBASH3B:
-
Ubiquitin Associated and SH3 Domain Containing B
- SYK:
-
Spleen tyrosine kinase
- HSPA1B:
-
Heat shock protein family A (Hsp70) member 1B
- ChIP:
-
Chromatin immunoprecipitation
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- DMSO:
-
Dimethyl sulfate
- PKC:
-
Protein Kinase C
- AP1:
-
Activator protein 1
- MAPK:
-
Mitogen-Activated Protein Kinase
- PGM:
-
Phosphoglycerate mutase-like/C-terminal histidine phosphatase
References
Tsygankov AY. TULA proteins in men, mice, hens, and lice: welcome to the family. Int J Mol Sci. 2023;24(11):9126.
Carpino N, Turner S, Mekala D, Takahashi Y, Zang H, Geiger TL, Doherty P, Ihle JN. Regulation of ZAP-70 activation and TCR signaling by two related proteins, Sts-1 and Sts-2. Immunity. 2004;20(1):37–46.
Carpino N, Kobayashi R, Zang H, Takahashi Y, Jou ST, Feng J, Nakajima H, Ihle JN. Identification, cDNA cloning, and targeted deletion of p70, a novel, ubiquitously expressed SH3 domain-containing protein. Mol Cell Biol. 2002;22(21):7491–500.
San Luis B, Sondgeroth B, Nassar N, Carpino N. Sts-2 is a phosphatase that negatively regulates zeta-associated protein (ZAP)-70 and T cell receptor signaling pathways. J Biol Chem. 2011;286(18):15943–54.
Concannon P, Onengut-Gumuscu S, Todd JA, Smyth DJ, Pociot F, Bergholdt R, Akolkar B, Erlich HA, Hilner JE, Julier C, et al. A human type 1 diabetes susceptibility locus maps to chromosome 21q22.3. Diabetes. 2008;57(10):2858–61.
Smyth DJ, Plagnol V, Walker NM, Cooper JD, Downes K, Yang JH, Howson JM, Stevens H, McManus R, Wijmenga C, et al. Shared and distinct genetic variants in type 1 diabetes and celiac disease. N Engl J Med. 2008;359(26):2767–77.
Zhernakova A, Stahl EA, Trynka G, Raychaudhuri S, Festen EA, Franke L, Westra HJ, Fehrmann RS, Kurreeman FA, Thomson B, et al. Meta-analysis of genome-wide association studies in celiac disease and rheumatoid arthritis identifies fourteen non-HLA shared loci. PLoS Genet. 2011;7(2): e1002004.
Chen YG, Ciecko AE, Khaja S, Grzybowski M, Geurts AM, Lieberman SM. UBASH3A deficiency accelerates type 1 diabetes development and enhances salivary gland inflammation in NOD mice. Sci Rep. 2020;10(1):12019.
Hoeller D, Crosetto N, Blagoev B, Raiborg C, Tikkanen R, Wagner S, Kowanetz K, Breitling R, Mann M, Stenmark H, et al. Regulation of ubiquitin-binding proteins by monoubiquitination. Nat Cell Biol. 2006;8(2):163–9.
Bertelsen V, Breen K, Sandvig K, Stang E, Madshus IH. The Cbl-interacting protein TULA inhibits dynamin-dependent endocytosis. Exp Cell Res. 2007;313(8):1696–709.
Feshchenko EA, Smirnova EV, Swaminathan G, Teckchandani AM, Agrawal R, Band H, Zhang X, Annan RS, Carr SA, Tsygankov AY. TULA: an SH3- and UBA-containing protein that binds to c-Cbl and ubiquitin. Oncogene. 2004;23(27):4690–706.
Wattenhofer M, Shibuya K, Kudoh J, Lyle R, Michaud J, Rossier C, Kawasaki K, Asakawa S, Minoshima S, Berry A, et al. Isolation and characterization of the UBASH3A gene on 21q22.3 encoding a potential nuclear protein with a novel combination of domains. Hum Genet. 2001;108(2):140–7.
Chen Y, Jakoncic J, Carpino N, Nassar N. Structural and functional characterization of the 2H-phosphatase domain of Sts-2 reveals an acid-dependent phosphatase activity. Biochemistry. 2009;48(8):1681–90.
Mikhailik A, Ford B, Keller J, Chen Y, Nassar N, Carpino N. A phosphatase activity of Sts-1 contributes to the suppression of TCR signaling. Mol Cell. 2007;27(3):486–97.
Chen X, Ren L, Kim S, Carpino N, Daniel JL, Kunapuli SP, Tsygankov AY, Pei D. Determination of the substrate specificity of protein-tyrosine phosphatase TULA-2 and identification of Syk as a TULA-2 substrate. J Biol Chem. 2010;285(41):31268–76.
Goyama S, Schibler J, Gasilina A, Shrestha M, Lin S, Link KA, Chen J, Whitman SP, Bloomfield CD, Nicolet D, et al. UBASH3B/Sts-1-CBL axis regulates myeloid proliferation in human preleukemia induced by AML1-ETO. Leukemia. 2016;30(3):728–39.
Lee ST, Feng M, Wei Y, Li Z, Qiao Y, Guan P, Jiang X, Wong CH, Huynh K, Wang J, et al. Protein tyrosine phosphatase UBASH3B is overexpressed in triple-negative breast cancer and promotes invasion and metastasis. Proc Natl Acad Sci U S A. 2013;110(27):11121–6.
Yao Y, Liu W, Gajendran B, Wang C, Zacksenhaus E, Sample KM, Varier KM, Hao X, Ben-David Y. Ubash3b promotes TPA-mediated suppression of leukemogenesis through accelerated downregulation of PKCdelta protein. Biochimie. 2021;184:8–17.
Asano Y, Trojanowska M. Phosphorylation of Fli1 at threonine 312 by protein kinase C delta promotes its interaction with p300/CREB-binding protein-associated factor and subsequent acetylation in response to transforming growth factor beta. Mol Cell Biol. 2009;29(7):1882–94.
Li YJ, Zhao X, Vecchiarelli-Federico LM, Li Y, Datti A, Cheng Y, Ben-David Y. Drug-mediated inhibition of Fli-1 for the treatment of leukemia. Blood Cancer J. 2012;2(1): e54.
Liu T, Yao Y, Zhang G, Wang Y, Deng B, Song J, Li X, Han F, **ao X, Yang J, et al. A screen for Fli-1 transcriptional modulators identifies PKC agonists that induce erythroid to megakaryocytic differentiation and suppress leukemogenesis. Oncotarget. 2017;8(10):16728–43.
Ben-David Y, Giddens EB, Bernstein A. Identification and map** of a common proviral integration site Fli-1 in erythroleukemia cells induced by Friend murine leukemia virus. Proc Natl Acad Sci U S A. 1990;87(4):1332–6.
Ben-David Y, Giddens EB, Letwin K, Bernstein A. Erythroleukemia induction by Friend murine leukemia virus: insertional activation of a new member of the ets gene family, Fli-1, closely linked to c-ets-1. Genes Dev. 1991;5(6):908–18.
Azimi A, Tuominen R, Costa Svedman F, Caramuta S, Pernemalm M, Frostvik Stolt M, Kanter L, Kharaziha P, Lehtio J, Hertzman Johansson C, et al. Silencing FLI or targeting CD13/ANPEP lead to dephosphorylation of EPHA2, a mediator of BRAF inhibitor resistance, and induce growth arrest or apoptosis in melanoma cells. Cell Death Dis. 2017;8(8): e3029.
Bonetti P, Testoni M, Scandurra M, Ponzoni M, Piva R, Mensah AA, Rinaldi A, Kwee I, Tibiletti MG, Iqbal J, et al. Deregulation of ETS1 and FLI1 contributes to the pathogenesis of diffuse large B-cell lymphoma. Blood. 2013;122(13):2233–41.
Chen N, Zhao G, Yan X, Lv Z, Yin H, Zhang S, Song W, Li X, Li L, Du Z, et al. A novel FLI1 exonic circular RNA promotes metastasis in breast cancer by coordinately regulating TET1 and DNMT1. Genome Biol. 2018;19(1):218.
Delattre O, Zucman J, Plougastel B, Desmaze C, Melot T, Peter M, Kovar H, Joubert I, de Jong P, Rouleau G, et al. Gene fusion with an ETS DNA-binding domain caused by chromosome translocation in human tumours. Nature. 1992;359(6391):162–5.
Kornblau SM, Qiu YH, Zhang N, Singh N, Faderl S, Ferrajoli A, York H, Qutub AA, Coombes KR, Watson DK. Abnormal expression of FLI1 protein is an adverse prognostic factor in acute myeloid leukemia. Blood. 2011;118(20):5604–12.
Liu T, **a L, Yao Y, Yan C, Fan Y, Gajendran B, Yang J, Li YJ, Chen J, Filmus J, et al. Identification of diterpenoid compounds that interfere with Fli-1 DNA binding to suppress leukemogenesis. Cell Death Dis. 2019;10(2):117.
Mhawech-Fauceglia P, Herrmann FR, Bshara W, Odunsi K, Terracciano L, Sauter G, Cheney RT, Groth J, Penetrante R, Mhawech-Fauceglia P. Friend leukaemia integration-1 expression in malignant and benign tumours: a multiple tumour tissue microarray analysis using polyclonal antibody. J Clin Pathol. 2007;60(6):694–700.
Sakurai T, Kondoh N, Arai M, Hamada J, Yamada T, Kihara-Negishi F, Izawa T, Ohno H, Yamamoto M, Oikawa T. Functional roles of Fli-1, a member of the Ets family of transcription factors, in human breast malignancy. Cancer Sci. 2007;98(11):1775–84.
Scheiber MN, Watson PM, Rumboldt T, Stanley C, Wilson RC, Findlay VJ, Anderson PE, Watson DK. FLI1 expression is correlated with breast cancer cellular growth, migration, and invasion and altered gene expression. Neoplasia (New York, NY). 2014;16(10):801–13.
Smeets MF, Chan AC, Dagger S, Bradley CK, Wei A, Izon DJ. Fli-1 overexpression in hematopoietic progenitors deregulates T cell development and induces pre-T cell lymphoblastic leukaemia/lymphoma. PLoS ONE. 2013;8(5): e62346.
Song W, Li W, Li L, Zhang S, Yan X, Wen X, Zhang X, Tian H, Li A, Hu JF, et al. Friend leukemia virus integration 1 activates the Rho GTPase pathway and is associated with metastasis in breast cancer. Oncotarget. 2015;6(27):23764–75.
Torlakovic EE, Slipicevic A, Florenes VA, Chibbar R, DeCoteau JF, Bilalovic N. Fli-1 expression in malignant melanoma. Histol Histopathol. 2008;23(11):1309–14.
Yan X, Yu Y, Li L, Chen N, Song W, He H, Dong J, Liu X, Cui J. Friend leukemia virus integration 1 is a predictor of poor prognosis of breast cancer and promotes metastasis and cancer stem cell properties of breast cancer cells. Cancer Med. 2018;7(8):3548–60.
Ben-David Y, Gajendran B, Sample KM, Zacksenhaus E. Current insights into the role of Fli-1 in hematopoiesis and malignant transformation. Cell Mol Life Sci. 2022;79(3):163.
Wang C, Sample KM, Gajendran B, Kapranov P, Liu W, Hu A, Zacksenhaus E, Li Y, Hao X, Ben-David Y. FLI1 induces megakaryopoiesis gene expression through WAS/WIP-dependent and independent mechanisms; implications for Wiskott-Aldrich syndrome. Front Immunol. 2021;12: 607836.
Sloan CA, Chan ET, Davidson JM, Malladi VS, Strattan JS, Hitz BC, Gabdank I, Narayanan AK, Ho M, Lee BT, et al. ENCODE data at the ENCODE portal. Nucleic Acids Res. 2016;44(D1):D726–732.
Motomura H, Seki S, Shiozawa S, Aikawa Y, Nogami M, Kimura T. A selective c-Fos/AP-1 inhibitor prevents cartilage destruction and subsequent osteophyte formation. Biochem Biophys Res Commun. 2018;497(2):756–61.
Wang X, Oates JC, Helke KL, Gilkeson GS, Zhang XK. Camptothecin and topotecan, inhibitors of transcription factor fli-1 and topoisomerase, markedly ameliorate lupus nephritis in (NZB x NZW)F1 mice and reduce the production of inflammatory mediators in human renal cells. Arthritis Rheumatol. 2021;73(8):1478–88.
Schutt SD, Wu Y, Kharel A, Bastian D, Choi HJ, Hanief Sofi M, Mealer C, McDaniel Mims B, Nguyen H, Liu C, et al. The druggable transcription factor Fli-1 regulates T cell immunity and tolerance in graft-versus-host disease. J Clin Invest. 2022;132(21): e143950.
Leveille E, Chan LN, Mirza AS, Kume K, Müschen M. SYK and ZAP70 kinases in autoimmunity and lymphoid malignancies. Cell Signal. 2022;94: 110331.
Sun S, Xue D, Chen Z, Ou-Yang Y, Zhang J, Mai J, Gu J, Lu W, Liu X, Liu W, et al. R406 elicits anti-warburg effect via syk-dependent and -independent mechanisms to trigger apoptosis in glioma stem cells. Cell Death Dis. 2019;10(5):358.
Chen B, Sheng D, Wang C, Liu W, Hu A, **ao X, Gajendran B, Gao J, Hu J, Sample KM, et al. FLI1 regulates inflammation-associated genes to accelerate leukemogenesis. Cell Signal. 2022;92: 110269.
Song D, Lian Y, Zhang L. The potential of activator protein 1 (AP-1) in cancer targeted therapy. Front Immunol. 2023;14:1224892.
Shaulian E. AP-1–The Jun proteins: Oncogenes or tumor suppressors in disguise? Cell Signal. 2010;22(6):894–9.
Somervaille TC, Cleary ML. PU.1 and Junb: suppressing the formation of acute myeloid leukemia stem cells. Cancer Cell. 2006;10(6):456–7.
Guan Y, Zhu X, Liang J, Wei M, Huang S, Pan X. Upregulation of HSPA1A/HSPA1B/HSPA7 and downregulation of HSPA9 were related to poor survival in colon cancer. Front Oncol. 2021;11: 749673.
Acknowledgements
Not applicable.
Funding
This research was funded by research grants from the National Natural Science Foundation of China (U1812403, 21867009, and 82260040), the Science and Technology Department of Guizhou Province grants (QKHJC-ZK [2022] YB297, QKHJC-ZK [2023] YB240) and the Key Laboratory of Chemistry for Natural Products of Guizhou Province and Chinese Academic of Sciences Research Grant (GZCNP202203Z) to XX and CW, the Guizhou Medical University Research Grant (RN21025) to BG.
Author information
Authors and Affiliations
Contributions
J.W., C.W., X.X., A.H., K.Y., Y.K., B.G., K.S. and, W.L. contributed to the conception, design of the study, as well as methodology, data acquisition and interpretation. W.L., X.X. and B.G. were involved in the statistical analysis and bioinformatics. J.W. and W.L. drafted the manuscript. Y.B.D., W.L. and E.Z. reviewed the manuscript critically. Y.B.D. supervised, conceived, funding acquisition and designed the study. All authors contributed to the interpretation of the findings, reviewed, edited and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, J., Wang, C., Hu, A. et al. FLI1 induces erythroleukemia through opposing effects on UBASH3A and UBASH3B expression. BMC Cancer 24, 326 (2024). https://doi.org/10.1186/s12885-024-12075-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-024-12075-2