Abstract
Background
Prostate cancer is a major health issue affecting the male population worldwide, and its etiology remains relatively unknown. As presented on the Gene Expression Profiling Interactive Analysis database, acetyl-CoA acetyltransferase 1 (ACAT1) acts as a prostate cancer-promoting factor. ACAT1 expression in prostate cancer tissues is considerably higher than that in normal tissues, leading to a poor prognosis in patients with prostate cancer. Here, we aimed to study the role of the ACAT1-fused in sarcoma (FUS) complex in prostate cancer and identify new targets for the diagnosis and treatment of the disease.
Methods
We conducted immunohistochemical analysis of 57 clinical samples and in vitro and in vivo experiments using a mouse model and plasmid constructs to determine the expression of ACAT1 in prostate cancer.
Results
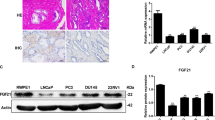
The relationship between the expression of ACAT1 and the Gleason score was significant. The expression of ACAT1 was higher in tissues with a Gleason score of > 7 than in tissues with a Gleason score of ≤7 (P = 0.0011). In addition, we revealed that ACAT1 can interact with the FUS protein.
Conclusions
In prostate cancer, ACAT1 promotes the expression of P62 and Nrf2 through FUS and affects reactive oxygen species scavenging. These effects are due to the inhibition of autophagy by ACAT1. That is, ACAT1 promotes prostate cancer by inhibiting autophagy and eliminating active oxygen species. The expression of ACAT1 is related to prostate cancer. Studying the underlying mechanism may provide a new perspective on the treatment of prostate cancer.
Similar content being viewed by others
Background
Prostate cancer is the second most frequently diagnosed malignancy and was the fifth leading cause of cancer-related deaths in men worldwide in 2020 [1, 2]. An increasing number of young men have been suffering from prostate cancer [3]. Therefore, the prevention and treatment of prostate cancer are essential. Commonly used treatment methods for prostate cancer include surgical treatment, non-invasive treatment, and androgen therapy [4,5,6]. With emerging treatment methods, such as immunotherapy and molecular therapy [7,8,9], the treatment of prostate cancer and the prognosis have improved. However, prostate cancer prevention and early treatment options are still research hotspots [10].
Acetyl-CoA acetyltransferase 1 (ACAT1) is an important enzyme [11,12,13,14,15,16,3].
In this study, we found that ACAT1 inhibited autophagy in prostate cells and exerted an ROS-scavenging effect. Both autophagy and increased ROS levels have certain tumor-inhibiting effects [27,28,29], and ACAT1 inhibits autophagy and eliminates intracellular ROS mainly by binding to FUS. FUS is an RNA-binding protein, which can promote the production of hnRNA in cells, thereby promoting the transcription of related genes [30]. In this study, we showed that FUS can promote the transcription of LC3B. LC3B is a key protein involved in autophagy. It mainly exists on the membrane of autophagosomes. When LC3A changes to LC3B, autophagosomes are formed. Furthermore, P62 binds to LC3B and autophagosomes on the membrane of a corpuscle; as the autophagosome enters the lysosome, it is degraded [31,32,33]. We believe that autophagy exerts cytotoxicity in prostate cancer. The results of the present study show that after binding to FUS, ACAT1 did not change FUS expression but inhibited FUS from entering the nucleus, thereby inhibiting the production of the autophagy-specific protein LC3B. We speculate that P62 accumulation might be caused by the damage that occurs in the later stages of autophagy [34], which prevents P62 from entering the autophagolysosome, and thereafter, its degradation. Nrf2 is located downstream of P62 [35, 36]. Nrf2 expression increased with the increase in P62 expression, and the active oxygen was scavenged.
Conclusions
Although the mechanism by which ACAT1 promotes prostate cancer has been demonstrated, the mechanism by which ACAT1 prevents FUS from entering the nucleus needs to be further explored. Furthermore, the underlying mechanism of how FUS affects LC3B transcription has not been elucidated and requires further investigation. Nonetheless, we demonstrated that the ACAT1-FUS complex plays a crucial role in prostate cancer development, which provides insights into the development of new therapeutic strategies by targeting or inhibiting the formation of this complex in prostate cancer.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- ACAT1:
-
Acetyl-CoA acetyltransferase 1
- ALS:
-
Amyotrophic lateral sclerosis
- FUS:
-
Fused in sarcoma
- GEPIA:
-
Gene expression profiling interactive analysis
- ROS:
-
Reactive oxygen species
- SPF:
-
Specific pathogen-free
References
Hu J, Han B, Huang J. Morphologic spectrum of neuroendocrine tumors of the prostate: An updated review. Arch Pathol Lab Med. 2020;144(320-5). https://doi.org/10.5858/arpa.2019-0434-RA.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(209-49). https://doi.org/10.3322/caac.21660.
Bleyer A, Spreafico F, Barr R. Prostate cancer in young men: An emerging young adult and older adolescent challenge (1). Cancer. 2020;126(46-57). https://doi.org/10.1002/cncr.32498.
Yassin A, AlRumaihi K, Alzubaidi R, Alkadhi S, Al AA. Testosterone, testosterone therapy and prostate cancer. Aging Male. 2019;22:219–27. https://doi.org/10.1080/13685538.2018.1524456.
Chow K, McCoy P, Stuchbery R, Corcoran NM, Hovens CM. Developments in oligometastatic hormone-sensitive prostate cancer. World J Urol. 2019;37(2549-55). https://doi.org/10.1007/s00345-018-2542-x.
Xu L, Mao X, Grey A, Scandura G, Guo T, Burke E, et al. Noninvasive detection of clinically significant prostate cancer using circulating tumor cells. J Urol. 2020;203(73-82). https://doi.org/10.1097/JU.0000000000000475.
Ziglioli F, Granelli G, Cavalieri D, Bocchialini T, Maestroni U. What chance do we have to decrease prostate cancer overdiagnosis and overtreatment? A narrative review. Acta Biomed. 2019;90(423-6). https://doi.org/10.23750/abm.v90i4.9070.
Cha HR, Lee JH, Ponnazhagan S. Revisiting immunotherapy: A focus on prostate cancer. Cancer Res. 2020;80(1615-23). https://doi.org/10.1158/0008-5472.CAN-19-2948.
Fay EK, Graff JN. Immunotherapy in prostate cancer. Cancers (Basel). 2020;12(1752). https://doi.org/10.3390/cancers12071752.
Yuan Y, Kishan AU, Nickols NG. Treatment of the primary tumor in metastatic prostate cancer. World J Urol. 2019;37(2597-606). https://doi.org/10.1007/s00345-018-2552-8.
Qian H, Zhao X, Yan R, Yao X, Gao S, Sun X, et al. Structural basis for catalysis and substrate specificity of human ACAT1. Nature. 2020;581(333-8). https://doi.org/10.1038/s41586-020-2290-0.
Ohshiro T, Matsuda D, Sakai K, Degirolamo C, Yagyu H, Rudel LL, et al. Pyripyropene A, an acyl-coenzyme A: cholesterol acyltransferase 2-selective inhibitor, attenuates hypercholesterolemia and atherosclerosis in murine models of hyperlipidemia. Arterioscler Thromb Vasc Biol. 2011;31(1108-15). https://doi.org/10.1161/ATVBAHA.111.223552.
Hartmann T, Kuchenbecker J, Grimm MO. Alzheimer’s disease: The lipid connection. J Neurochem. 2007;103(Suppl 1):159–70. https://doi.org/10.1111/j.1471-4159.2007.04715.x.
Jiang Y, Sun A, Zhao Y, Ying W, Sun H, Yang X, et al. Proteomics identifies new therapeutic targets of early-stage hepatocellular carcinoma. Nature. 2019;567(257-61). https://doi.org/10.1038/s41586-019-0987-8.
Li J, Gu D, Lee SS, Song B, Bandyopadhyay S, Chen S, et al. Abrogating cholesterol esterification suppresses growth and metastasis of pancreatic cancer. Oncogene. 2016;35(6378-88). https://doi.org/10.1038/onc.2016.168.
Yue S, Li J, Lee SY, Lee HJ, Shao T, Song B, et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014;19(393-406). https://doi.org/10.1016/j.cmet.2014.01.019.
Yang W, Bai Y, **ong Y, Zhang J, Chen S, Zheng X, et al. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature. 2016;531(651-5). https://doi.org/10.1038/nature17412.
Gu L, Zhu Y, Lin X, Tan X, Lu B, Li Y. Stabilization of FASN by ACAT1-mediated GNPAT acetylation promotes lipid metabolism and hepatocarcinogenesis. Oncogene. 2020;39(2437-49). https://doi.org/10.1038/s41388-020-1156-0.
Ayyagari VN, Wang X, Diaz-Sylvester PL, Groesch K, Brard L. Assessment of acyl-CoA cholesterol acyltransferase (ACAT-1) role in ovarian cancer progression-an in vitro study. PLoS One. 2020;15(e0228024). https://doi.org/10.1371/journal.pone.0228024.
Cui W, Luo W, Zhou X, Lu Y, Xu W, Zhong S, et al. Dysregulation of ketone body metabolism is associated with poor prognosis for clear cell renal cell carcinoma patients. Front. Oncol. 2019;9(1422). https://doi.org/10.3389/fonc.2019.01422.
Guan J, Jiang X, Gai J, Sun X, Zhao J, Li J, et al. Sirtuin 5 regulates the proliferation, invasion and migration of prostate cancer cells through acetyl-CoA acetyltransferase 1. J Cell Mol Med. 2020;24(14039-49). https://doi.org/10.1111/jcmm.16016.
Errichelli L, Dini Modigliani S, Laneve P, Colantoni A, Legnini I, Capauto D, et al. FUS affects circular RNA expression in murine embryonic stem cell-derived motor neurons. Nat Commun. 2017;8(14741). https://doi.org/10.1038/ncomms14741.
Ederle H, Dormann D. TDP-43 and FUS en route from the nucleus to the cytoplasm. FEBS Lett. 2017;591(1489-507). https://doi.org/10.1002/1873-3468.12646.
Picchiarelli G, Demestre M, Zuko A, Been M, Higelin J, Dieterlé S, et al. FUS-mediated regulation of acetylcholine receptor transcription at neuromuscular junctions is compromised in amyotrophic lateral sclerosis. Nat Neurosci. 2019;22(1793-805). https://doi.org/10.1038/s41593-019-0498-9.
Ghanbarpanah E, Kohanpour MA, Hosseini-Beheshti F, Yari L, Keshvari M. Structure and function of FUS gene in prostate cancer. Bratisl Lek Listy. 2018;119(660-3). https://doi.org/10.4149/BLL_2018_118.
Sun X, Wang S, Gai J, Guan J, Li J, Li Y, et al. SIRT5 promotes cisplatin resistance in ovarian cancer by suppressing DNA damage in a ROS-dependent manner via regulation of the Nrf2/HO-1 pathway. Front. Oncol. 2019;9(754). https://doi.org/10.3389/fonc.2019.00754.
Jiang X, Huang Y, Liang X, Jiang F, He Y, Li T, et al. Metastatic prostate cancer-associated P62 inhibits autophagy flux and promotes epithelial to mesenchymal transition by sustaining the level of HDAC6. Prostate. 2018;78(426-34). https://doi.org/10.1002/pros.23487.
Chen W, Li P, Liu Y, Yang Y, Ye X, Zhang F, et al. Isoalantolactone induces apoptosis through ROS-mediated ER stress and inhibition of STAT3 in prostate cancer cells. J Exp Clin Cancer Res. 2018;37(309). https://doi.org/10.1186/s13046-018-0987-9.
Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. 2018;80(50-64). https://doi.org/10.1016/j.semcdb.2017.05.023.
Wang J, Choi JM, Holehouse AS, Ho L, Zhang X, Jahnel M, et al. A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell. 2018;174:688–699.e16. https://doi.org/10.1016/j.cell.2018.06.006.
Bresciani A, Spiezia MC, Boggio R, Cariulo C, Nordheim A, Altobelli R, et al. Quantifying autophagy using novel LC3B and p62 TR-FRET assays. PLoS One. 2018;13(e0194423). https://doi.org/10.1371/journal.pone.0194423.
Zaffagnini G, Savova A, Danieli A, Romanov J, Tremel S, Ebner M, et al. p62 filaments capture and present ubiquitinated cargos for autophagy. EMBO J. 2018;37(e98308). https://doi.org/10.15252/embj.201798308.
Goode A, Butler K, Long J, Cavey J, Scott D, Shaw B, et al. Defective recognition of LC3B by mutant SQSTM1/p62 implicates impairment of autophagy as a pathogenic mechanism in ALS-FTLD. Autophagy. 2016;12:1094–104. https://doi.org/10.1080/15548627.2016.1170257.
Langer R, Neppl C, Keller MD, Schmid RA, Tschan MP, Berezowska S. Expression analysis of autophagy related markers LC3B, p62 and HMGB1 indicate an autophagy-independent negative prognostic impact of high p62 expression in pulmonary squamous cell carcinomas. Cancers (Basel). 2018;10(281). https://doi.org/10.3390/cancers10090281.
Jena KK, Kolapalli SP, Mehto S, Nath P, Das B, Sahoo PK, et al. TRIM16 controls assembly and degradation of protein aggregates by modulating the p62-NRF2 axis and autophagy. EMBO J. 2018;37(e98358). https://doi.org/10.15252/embj.201798358.
Lee DH, Park JS, Lee YS, Han J, Lee DK, Kwon SW, et al. SQSTM1/P62 activates NFE2L2/NRF2 via ULK1-mediated autophagic KEAP1 degradation and protects mouse liver from lipotoxicity. Autophagy. 2020;16:1949–73. https://doi.org/10.1080/15548627.2020.1712108.
Acknowledgments
We thank Shanghai Wcgene Technology Co., Ltd. for providing the autophagy PCR array.
Funding
This work was supported by the National Natural Science Foundation of China (grant number 81874214) and National Natural Science Foundation of China Youth Foud (grant number 81702269). .
Author information
Authors and Affiliations
Contributions
JG designed the study, conducted the experiments, acquired and analyzed the data, and wrote the manuscript. XJ, YG, WZ, JL, YL, MC, and LF conducted the experiments and acquired the data. YZ and QL were responsible for the conception and supervision of the study and wrote the manuscript. All authors corrected the drafts and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of China Medical University, and all patients signed informed consent forms. The study was carried out in compliance with the ARRIVE guidelines, and all procedures in animal experiments followed the ethical standards. All animal experiments were approved by the Institutional Animal Care and Use Committee of China Medical University and adhered to the guidelines for the care and use of laboratory animals issued by the China Animal Research Council.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
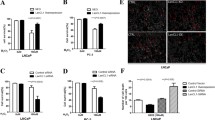
ACAT1 is an oncogene in prostate cancer. A. Edu assay showing changes in the proliferation abilities of LNCaP and PC3 cells after an increase in ACAT1 expression. *P < 0.05, **P < 0.01. B. Transwell cell migration assay showing changes in the migration ability of LNCaP and PC3 cells after an increase in FUS expression. *P < 0.05, **P < 0.01.
Additional file 2: Fig. S2.
Associated expression of ACAT1 in prostate cancer cells. A. Expression of ACAT1 and FUS in four common prostate cancer cell lines (LNCaP, PC3, DU145, and 22RV1). The statistical analysis of gray values is shown. B. Expression of ACAT1 in the nucleus and cytoplasm. The statistical analysis of gray values is shown. *P < 0.05, **P < 0.01. C. In q-PCR assay, the mRNA levels of Nrf2 and P62 did not change significantly after an increase in ACAT1 expression in LNCaP cells and PC3 cells. D. In q-PCR assay, ACAT1 and FUS expression was increased in LNCaP and PC3 cells at the same time, whereas the mRNA levels of Nrf2 and P62 did not change significantly. E. Changes in autophagy-related protein expression after a decrease in ACAT1 expression. The statistical analysis of gray values is shown. *P < 0.05, **P < 0.01.
Additional file 3: Fig. S3.
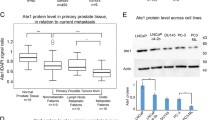
Changes in the FUS protein level when ACAT1 and FUS expression is elevated simultaneously. A. Western blot showing changes in the FUS protein level in LNCaP cells when ACAT1 and FUS expression was elevated simultaneously. B. Western blot showing changes in the FUS protein level in PC3 cells when ACAT1 and FUS expression was elevated simultaneously.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Guan, J., Jiang, X., Guo, Y. et al. Autophagy inhibition and reactive oxygen species elimination by acetyl-CoA acetyltransferase 1 through fused in sarcoma protein to promote prostate cancer. BMC Cancer 22, 1313 (2022). https://doi.org/10.1186/s12885-022-10426-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-10426-5