Abstract
Background
There is no clear consensus on the benefits of adjuvant chemotherapy for tumor-node-metastasis (TNM) stage T1 (T1N0M0) breast cancer (BC). Our study investigated the effects of adjuvant chemotherapy on T1N0M0 BC patients.
Methods
Seventy-five thousand one hundred thirty-nine patients diagnosed with T1N0M0 BC were selected from the Surveillance, Epidemiology, and End Results (SEER) database. Multivariate Cox analyses were performed to investigate the effects of adjuvant chemotherapy on T1a, T1b, and T1cN0M0 BC, including various tumor grades, and four molecular subtypes. Propensity score matching (PSM) was used to eliminate confounding factors and further compare the results between adjuvant chemotherapy and no adjuvant chemotherapy. Additionally, 545 T1N0M0 BC patients treated at the Northern Jiangsu People’s Hospital were included as an independent external validation cohort. Univariate and multivariate Cox analyses were used to confirm the effects of adjuvant chemotherapy in T1a, T1b, and T1cN0M0 BC. Survival curves for the different tumor grades and molecular subtypes were plotted using the Kaplan–Meier method.
Results
Adjuvant chemotherapy demonstrated a statistically significant improvement in overall survival (OS) in T1b and T1c BC, but not in T1a BC. Within T1b BC, adjuvant chemotherapy was found to have effects on grade III, and hormone receptor + (HoR +)/human epidermal growth factor receptor 2 + (HER2 +), HoR-/HER2 + , and HoR-/HER2- molecular subtypes, respectively. Adjuvant chemotherapy was beneficial to OS for grade II/III and T1c BC. Identical results were obtained after PSM. We also obtained similar results with external validation cohort, except that adjuvant chemotherapy made a difference in grade II and T1b BC of the external validation dataset.
Conclusions
Partial T1N0M0 BC patients with grade III T1bN0M0, patients with tumor grade II and III T1cN0M0, and excluding those with HoR + /HER2- subtype tumors, could obtain OS benefits from adjuvant chemotherapy.
Similar content being viewed by others
Background
Breast cancer (BC) is the most commonly occurring malignancy in women and the leading cause of cancer-related death among women worldwide [1]. The incidence of early-stage BC has increased over the recent decades due to the widespread use of advanced diagnostic imaging and longer life expectancy [2,3,4,5]. Small tumors without lymph node infiltration have been given much attention by physicians. These patients are considered to have a good prognosis, even though they undergo surgery without adjuvant therapy. T1N0M0 BC includes tumors smaller than 2 cm without node involvement. These tumors are subdivided into three groups: T1a (≤ 0.5 cm), T1b (> 0.5 cm but ≤ 1.0 cm), and T1c (> 1.0 cm but ≤ 2.0 cm) [6]. According to the seventh edition of the American Joint Committee on Cancer, T1N0M0 BC has been reported to have a relatively low risk of death and recurrence [7, 8].
BC is a heterogeneous disease with distinct response to therapeutics, and consists of four different molecular subtypes, namely, luminal A, luminal B, HER2 + , and triple-negative breast cancer (TNBC) [9]. Considering the results of international reviews, adjuvant therapy for BC can reduce deaths by approximately 25% across all the risk groups [10]. Adjuvant systematic therapy includes chemotherapy, endocrine therapy, and targeted therapy. Although adjuvant chemotherapy is an effective treatment, it is known to cause short-term and long-term side effects that are toxic and could result in death [11]. Patients with early-stage BC have been reported to achieve only a small absolute percentage of survival benefit from adjuvant chemotherapy [12]. Therefore, it is crucial to identify the patients with early-stage BC that may benefit from adjuvant chemotherapy. When considering adjuvant chemotherapy, it is important to weigh the possible risks against the benefits. Adjuvant chemotherapy can reduce the risk of tumor recurrence and death, but it also increases the damage caused by its toxic side-effects and increases the medical expenses. Nevertheless, without further evidence, the possible benefits of adjuvant chemotherapy for early-stage BC are controversial. Therefore, our study aimed to evaluate the effects of adjuvant chemotherapy on the overall survival (OS) of T1N0M0 BC patients.
Materials and methods
Data acquisition and patient selection
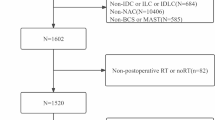
The Surveillance, Epidemiology, and End Results (SEER) database was established in 1973 by the National Cancer Institute and is one of the largest tumor registration database. Data from 18 registries of the SEER program (2010 to 2014) was used to identify T1N0M0 female BC patients. Breast cancer patients that met the following criteria were excluded: (a) did not undergo surgery; (b) had a history of ductal carcinoma in situ or lobular carcinoma in situ; (c) had a history of other malignancy /chronic diseases; (d) participated for < 3 months of follow-up; and (e) had incomplete or missing clinicopathological data.
We collected the data of 75,139 early-stage BC patients from the SEER database. The same inclusion and exclusion criteria were applied to the external validation cohort, consisting of patients treated at the Northern Jiangsu People’s Hospital from 2010 to 2015. Finally, 545 female T1N0M0 BC patients were included in the study from the external validation cohort.
The following data of each patient were gathered: patient number, age, death status (yes/no), follow-up time, tumor size, grade, estrogen receptor (ER) status, progesterone receptor (PR) status, human epidermal growth factor receptor‐2 (HER‐2) status, surgical method, history of adjuvant chemotherapy, and history of radiation therapy. Because chemotherapy-related toxicities could lead to death, we chose OS as the endpoint instead of BC-specific survival [13]. An age of 60 years was chosen as the threshold for distinguishing young and old patients [14].
SEER methods
A cohort of 75,139 eligible early-stage BC patients was identified from the SEER database. We conducted a descriptive analysis of the baseline clinical features of eligible patients and used the chi-square test to compare the characteristics of patients among the three groups. Multivariate Cox regression analysis was performed to explore whether adjuvant chemotherapy was a prognostic factor for T1a, T1b, and T1c BC patients. We also performed a multivariate Cox regression analysis of diverse tumor grades and molecular subtypes. To further confirm the specific role of adjuvant chemotherapy for patients in the three groups and four molecular subtypes, a multivariate Cox regression analysis of tumor grades was performed. Furthermore, propensity score matching (PSM) was performed to balance the disparities between adjuvant chemotherapy and no adjuvant chemotherapy. The variables considered in the PSM analysis for adjuvant chemotherapy status were tumor grade, surgery type, radiation record, molecular subtype, and age. Following PSM, multivariate Cox regression analysis was repeated in order to obtain more accurate results, and to assess the usefulness of adjuvant chemotherapy for the different tumor grades and molecular subtypes of the three groups.
External validation methods
A total of 545 T1N0M0 female BC patients were included in the external validation cohort from the Northern Jiangsu People’s Hospital. Because of the lack of data, we used the univariate Cox regression analysis to identify the significant variables and remove some inconsequential parameters. These meaningful indicators were incorporated into the multivariate Cox regression analysis to further evaluate the three groups. In each of the three groups, Kaplan–Meier survival curves of the tumor grades and molecular subtypes were plotted and the estimated log-rank test results were used to compare the control group (no adjuvant chemotherapy) and the experimental group (adjuvant chemotherapy).
Statistical methods
Data analyses were performed using the R software version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria) and SPSS version 19.0 (IBM Corporation, Armonk, NY, USA). PSM was calculated using multivariate logistic regression, and the propensity score was constructed using 1:1 nearest-neighbor matching within calipers (0.005), without replacement [15]. Two-tailed test with a p-value (P) < 0.05 was considered statistically significant.
Results
SEER results
The median follow-up time was 51 months, as calculated by the reverse Kaplan–Meier method. As shown in Table S1, a total of 75,139 T1N0M0 BC patients were divided into three groups: T1a (n = 10,073); T1b (n = 24,951); and T1c (n = 40,115). T1cN0M0 BC patients tended to have worse differentiated tumor grades than the other groups and often received adjuvant chemotherapy. There were notable statistical differences between the groups (P < 0.01). The outcomes of multivariate Cox proportional hazard analyses of T1, T1a, T1b, and T1c are presented in Table S2. Adjuvant chemotherapy was effective for T1b (hazard ratio (HR), 0.72; 95% confidence interval (CI), 0.59–0.88; P < 0.0001) and T1c (HR, 0.54; 95% CI, 0.48–0.60; P < 0.0001). Compared to the other subtypes, HoR + /HER2- BC subtype had a better prognosis with T1b and T1c. Patients treated with radiotherapy had more favorable survival rates (P < 0.0001). Multivariate Cox analyses (Table S3) showed that patients that underwent breast-conserving surgery combined with radiotherapy experienced a longer OS than patients that underwent breast-conserving surgery or total mastectomy or modified radical mastectomy alone (P < 0.0001). As expected, larger tumors were strong predictors of worse OS. Multivariate Cox regression analysis of the four molecular subtypes and tumor grades of T1a are presented in Tables S4 and S5. Adjuvant chemotherapy did not have a role in molecular subtypes HoR + /HER2- (P = 0.11), HoR + /HER2 + (P = 0.36), HoR-/HER2 + (P = 0.22), and HoR-/HER2- (P = 0.20) and tumor grades I (P = 0.78), II (P = 0.23), and III (P = 0.68). Multivariate Cox regression analysis of the four molecular subtypes and tumor grades of T1b are presented in Tables S6 and S7. There were no survival benefits with adjuvant chemotherapy in T1bN0M0 BC patients with HoR + /HER2- (P = 0.67), grade I (P = 0.41), and grade II (P = 0.11) tumors. There were statistically significant survival benefits with adjuvant chemotherapy in T1bN0M0 BC patients with HoR + /HER2 + (HR, 0.41; 95% CI, 0.25–0.69; P < 0.0001), HoR-/HER2 + (HR, 0.50; 95% CI, 0.25–1.02; P = 0.01), HoR-/HER2- (HR, 0.53; 95% CI, 0.36–0.78; P < 0.0001), and grade III (HR, 0.52; 95% CI, 0.38–0.70; P < 0.0001) tumors, as compared to patients that did not receive any adjuvant chemotherapy. T1c patients that received adjuvant chemotherapy appeared to have OS benefits compared to their counterparts, except for those with grade I (P = 0.10) (Tables S8 and S9). Multivariate Cox regression analyses of tumor grades of the T1a group and molecular subtype subgroups are presented in Tables S10, S11, S12, and S13. Not surprisingly, adjuvant chemotherapy did not improve the OS of T1a patients. However, adjuvant chemotherapy decreased the survival time for grade II (HR, 3.23; 95% CI, 1.81–5.78; P < 0.0001) and grade III (HR, 3.26; 95% CI, 1.25–8.48; P = 0.02) T1a patients with HoR + /HER2- subtype. Adjuvant chemotherapy led to accelerated death in grade I T1b patients with HoR + /HER2- (HR, 1.62; 95% CI, 1.02–2.57; P = 0.04) (Tables S14, S15, S16, and S17). Adjuvant chemotherapy also significantly enhanced the OS of grade III (HR, 0.36; 95% CI, 0.16–0.80; P = 0.01) T1b patients with HoR + /HER2 + , HoR-/HER2 + (HR, 0.34; 95% CI, 0.14–0.84; P = 0.02), and HoR-/HER2- (HR, 0.54; 95% CI, 0.34–0.85; P = 0.01). However, adjuvant chemotherapy was not useful for grade I T1c patients with HoR + /HER2- (P = 0.20), HoR + /HER2 + (P = 0.28), HoR-/HER2 + (P = 0.41), and HoR-/HER2- (P = 0.67) (Tables S18, S19, S20, and S21). Tables 1, 2 and 3 summarize the association of adjuvant chemotherapy with other variables. The results of the adjuvant chemotherapy group were clearly contrary to that of the no adjuvant chemotherapy group of the entire cohort (P < 0.01). After PSM, the parameters of the above two groups were similar in the matched cohort. Consequently, these matched data were used for further analyses and validation. In the matched cohort, adjuvant chemotherapy was statistically significant for T1b (HR, 0.44; 95% CI, 0.38–0.51; P < 0.001) and T1c (HR, 0.47; 95% CI, 0.41–0.54; P < 0.001) (Table 4). Similarly, T1a patients of the matched cohort (Tables 5 and 6) with tumor grades I (P = 0.27), II (P = 0.99), and III (P = 0.57) and molecular subtypes HoR + /HER2- (P = 0.66), HoR + /HER2 + (P = 0.20), HoR/HER2 + (P = 0.27), and HoR-/HER2- (P = 0.48) did not obtain OS benefit from adjuvant chemotherapy. Furthermore, T1b patients with HoR + /HER2 + (HR, 0.38; 95% CI, 0.21–0.66; P < 0.01), HoR-/HER2 + (HR, 0.51; 95% CI, 0.23–1.15; P = 0.01), HoR-/HER2- (HR, 0.44; 95% CI, 0.28–0.67; P < 0.0001) and grade III (HR, 0.51; 95% CI, 0.36–0.72; P < 0.0001), that received adjuvant chemotherapy, showed OS benefits (Tables 7 and 8). T1c patients that received adjuvant chemotherapy, appeared to obtain OS benefits compared to their counterparts, except for those with grade I (P = 0.33) of the matched cohort (Tables 9 and 10).
External validation results
The median follow-up time was 60 months, as calculated by the reverse Kaplan–Meier method. The external validation cohort consisted of 545 early-stage BC patients and was comprised of three groups: T1a (n = 98), T1b (n = 130), and T1c (n = 317) (Table S22). Larger tumors tended to have worse differentiated tumor grades and often received adjuvant chemotherapy (P < 0.01). Results of the univariate and multivariate Cox regression analyses of T1a, T1b, and T1c are presented in Tables S23, S24, and S25. T1a early-stage BC patients did not benefit from adjuvant chemotherapy (P = 0.47). Nevertheless, patients with T1b (HR, 0.02; 95% CI, 0.00–0.09; P < 0.0001) and T1c (HR, 0.06; 95% CI, 0.03–0.11; P < 0.0001) BC had longer survival with adjuvant therapy. Kaplan–Meier survival curves were plotted for tumor grades and molecular subtypes: T1a (Figure S1), T1b (Figure S2), and T1c (Figure S3). All statistically significant results are summarized in Figs. 1 and 2. For T1a BC patients, adjuvant chemotherapy was ineffective for those with grade I (P = 0.43), grade II (p = 0.25), HoR + /HER2- (P = 0.75), HoR + /HER2 + (P = 0.26), and HoR-/HER2 + (P = 1). Grade III and triple-negative BC groups could not be plotted due to limited data available for T1a. For patients with grade II (P < 0.0001), grade III (P < 0.01), HoR + /HER2 + (P < 0.01), HoR-/HER2 + (P < 0.0001), and HoR/HER2- (P < 0.0001) T1b BC (Fig. 1A, B, C, D and E), adjuvant chemotherapy had beneficial effects on OS. Adjuvant chemotherapy improved OS (P < 0.0001) for patients with T1c BC (Fig. 2A, B, C, D, E, and F), but not for those with grade I (P = 0.07).
Statistically significant Kaplan–Meier survival curves of the adjuvant chemotherapy and no adjuvant chemotherapy groups according to the grades and molecular subtypes of T1bN0M0 breast cancer patients treated at the Northern Jiangsu People’s Hospital. A T1b Grade II; B: T1b Grade III; C T1b HoR + HER2 + ; D T1b HoR- HER2 + ; E T1b HoR- HER2-. Abbreviations: HoR, hormone receptor; HER2, human epidermal growth factor receptor 2
Statistically significant Kaplan–Meier survival curves of the adjuvant chemotherapy and no adjuvant chemotherapy groups according to the grades and molecular subtypes of T1cN0M0 breast cancer patients treated at the Northern Jiangsu People’s Hospital. A T1c Grade II; B T1c Grade III; C T1c HoR + HER2-; D T1c HoR + HER2 + ; E T1c HoR- HER2 + ; F T1c HoR- HER2-. Abbreviations: HoR, hormone receptor; HER2, human epidermal growth factor receptor 2
Discussion
In spite of the dramatic increase in the number of early-stage BC patients [3,4,5, 16], the role of adjuvant chemotherapy in T1N0M0 BC remains controversial. Therefore, it is imperative to establish a safe, specific, and effective adjuvant chemotherapy strategy to guide treatment and improve the prognosis of these patients. In addition to creating the strategy, we utilized PSM and external validation dataset to verify the association between adjuvant chemotherapy and OS in T1N0M0 BC patients.
Adjuvant chemotherapy is recognized as a primary systematic adjuvant modality. However, it negatively influences survival and reduces the quality of life due to its short-term toxicities, including alopecia, nausea, vomiting, and fatigue, and potential long-term side-effects, including myelosuppression, cardiovascular toxicity, neurotoxicity, marrow neoplasm, and cessation of menses and fertility [17,18,19,20]. Early-stage BC patients are expected to survive their cancer diagnosis. As adjuvant chemotherapy associated toxicity could cause death, it is better to consider OS instead of BC-specific mortality as an end-point [13, 21]. Furthermore, our study suggests that adjuvant chemotherapy possibly accelerates death for some HoR + /HER2- T1aN0M0, and T1bN0M0 patients.
Postmastectomy radiation therapy is widely considered to reduce the risk of local recurrence and mortality, especially in patients with locally advanced tumors, as these patients are at a high risk due to large tumors and axillary lymph node involvement [22,23,24]. However, the majority of T1N0M0 BC patients prefer to undergo breast-conserving surgery instead of mastectomy. Adjuvant radiotherapy is a locoregional treatment that is often combined with breast-conserving surgery to achieve local control benefits and OS advantages [25,26,27,28,29,30]. These results are consistent with the results from our study.
The results from the SEER database were in contradiction to the results obtained from the external validation cohort. The external validation results indicated that patients with grade II T1bN0M0 could acquire survival benefit from adjuvant chemotherapy. However, this was not observed in the results from the SEER database. There are two explanations for this phenomenon. Firstly, the data used for external validation were relatively limited, Therefore, inevitable deviations might have occurred during the statistical analyses. Secondly, the two cohorts of data were derived from China and the United States, respectively. Admittedly, the factors that affect a patient’s lifetime vary from country to country and are influenced by cultural barriers, ethnic differences, and genetics [31]. Consequently, the final conclusions refer to the results obtained from the SEER database.
The results from our study, the guidelines of the National Comprehensive Cancer Network (NCCN), and the guidelines of the St. Gallen International BC Conference (BCC) are nearly identical [32, 33]. The NCCN suggests the following:
-
1.
For node-negative HoR + /HER2- BC, if the tumor is 0.5 cm or smaller, adjuvant chemotherapy is not recommended. If the tumor is larger than 0.5 cm, then performing a 21-gene reverse-transcription polymerase chain reaction assay (Oncotype DX) is strongly recommended [34,35,36].
-
a
If the recurrence score is ≥ 31, the risk of recurrence is high and adjuvant chemotherapy is recommended.
-
b
If the recurrence score is between 26–30, the risk of recurrence is moderate and the decision to perform adjuvant chemotherapy is based on other clinical factors.
-
c
If the recurrence score is < 26, the risk of recurrence is low and adjuvant chemotherapy is not recommended.
-
a
-
2.
For node-negative HoR + /HER2 + BC, if the tumor is 1.0 cm or smaller, it is unclear whether adjuvant chemotherapy is required. However, adjuvant chemotherapy is recommended for T1a category 2B, which means that there is an NCCN consensus that intervention is appropriate based on lower-level evidence. If the tumor is larger than 1.0 cm, adjuvant chemotherapy is recommended.
-
3.
For node-negative HoR-/HER2 + BC, adjuvant chemotherapy is recommended. Also, adjuvant chemotherapy is recommended for category 2B when the tumor is smaller than 0.5 cm.
-
4.
For node-negative HoR-/HER2- BC, if the tumor is smaller than 0.5 cm, adjuvant chemotherapy is not recommended. However, adjuvant chemotherapy is necessary for all other cases.
The BCC guidelines are different but somewhat similar to the NCCN guidelines [33]. Routine adjuvant chemotherapy is not recommended for T1aN0M0 BC; this is similar to the results from our study. The BCC panel recommends adjuvant chemotherapy for HER2 + and triple-negative BC (TNBC) stage T1bN0M0 and higher. For ER + /HER2- T1N0M0 BC, regardless of luminal-A-like qualities (strongly ER + and PR + , HER2-, with lower grade and proliferation markers) or luminal-B-like tumors, the BCC panel does not recommend adjuvant chemotherapy for patients with low genomic risk scores, according to the Oncotype DX and 70-gene signature tests (MammaPrint) [37,38,39,40]. Additionally, the European Society for Medical Oncology guidelines are in agreement with the St. Gallen guidelines regarding adjuvant chemotherapy for early-stage BC [41].
In our opinion, which is also supported by the St. Gallen guidelines, adjuvant chemotherapy should not be performed for T1aN0M0 BC patients. For HoR + /HER2- T1bN0M0 and T1cN0M0 BC, adjuvant chemotherapy is recommended for grade II and grade III T1cN0M0 BC when no genetic signature test has been performed or when the 21-gene assay indicates a medium risk. If the conditions are suitable, we propose to perform genetic testing for these patients, in accordance with the guidelines. For the other three molecular subtypes, adjuvant chemotherapy is recommended for stage T1bN0M0 and higher; this is also mentioned in the St. Gallen guidelines. Previous retrospective studies have demonstrated the survival benefits of adjuvant chemotherapy for patients with T1cN0M0 TNBC [42,43,44]. We incorporated tumor grade, which is an independent prognostic indicator, to assess the effects of adjuvant chemotherapy [45,46,47]. Patients, including those with TNBC, can be exempt from adjuvant chemotherapy if they have grade I/II T1bN0M0 and grade I T1cN0M0 BC [48].
Our study has several limitations. Firstly, the SEER database lacked information regarding the genetic background of the patients such as the 21-gene assay and schemes, and data regarding the therapies given to the patients, including details about the dosages of adjuvant chemotherapy and endocrine therapies. Secondly, this study lacked data regarding the muscle mass of the patients. Previous studies reported that chemotherapy could increase the hematological toxicity of BC patients with a low muscle mass, which might further affect their OS [49, 50]. Thirdly, because the endpoint was OS, age was a significant factor that could not be included to evaluate the effects of adjuvant chemotherapy. Fourthly, because we used a retrospective cohort population, inevitable selection bias might have affected the conclusions. Further large-scale, prospective, randomized, controlled clinical trials are warranted to accurately identify the outcomes.
Conclusions
Our study found that adjuvant chemotherapy is not beneficial and might even be detrimental to T1aN0M0 BC patients. Moreover, adjuvant chemotherapy is recommended for patients with tumor grade III T1bN0M0 and grade II/III T1cN0M0 BC, but not to patients with HoR + /HER2- BC. Regarding the molecular subtype HoR + /HER2-, in the absence of genetic testing, adjuvant chemotherapy is recommended for tumor grade II and grade III T1cN0M0 BC. However, further randomized, controlled clinical trials are needed to confirm these results.
Availability of data and materials
The data that support the findings of this study are available from the corresponding authors upon reasonable request. The dataset from SEER database generated and/or analyzed during the current study are available in the SEER dataset repository (https://seer.cancer.gov/data/).
Abbreviations
- BC:
-
Breast cancer
- SEER:
-
Surveillance, Epidemiology, and End Results
- PSM:
-
Propensity score matching
- OS:
-
Overall survival
- HoR:
-
Hormone receptor
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- HER‐2:
-
Human epidermal growth factor receptor‐2
- HR:
-
Hazard ratio
- NCCN:
-
National Comprehensive Cancer Network
- BCC:
-
Breast Cancer Conference
- TNBC:
-
Triple-negative breast cancer
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34.
Welch HG, Prorok PC, O’Malley AJ, Kramer BS. Breast-Cancer Tumor Size, Overdiagnosis, and Mammography Screening Effectiveness. N Engl J Med. 2016;375(15):1438–47.
Schootman M, Jeffe D, Reschke A, Aft R. The full potential of breast cancer screening use to reduce mortality has not yet been realized in the United States. Breast Cancer Res Treat. 2004;85(3):219–22.
Bhoo-Pathy N, Subramaniam S, Taib NA, Hartman M, Alias Z, Tan GH, Ibrahim RI, Yip CH, Verkooijen HM. Spectrum of very early breast cancer in a setting without organised screening. Br J Cancer. 2014;110(9):2187–94.
Marshall SF, Clarke CA, Deapen D, Henderson K, Largent J, Neuhausen SL, Reynolds P, Ursin G, Horn-Ross PL, Stram DO, et al. Recent breast cancer incidence trends according to hormone therapy use: the California Teachers Study cohort. Breast Cancer Res. 2010;12(1):R4.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–4.
Rosen PR, Groshen S, Saigo PE, Kinne DW, Hellman S. A long-term follow-up study of survival in stage I (T1N0M0) and stage II (T1N1M0) breast carcinoma. J Clin Oncol. 1989;7(3):355–66.
Lee AK, Loda M, Mackarem G, Bosari S, DeLellis RA, Heatley GJ, Hughes K. Lymph node negative invasive breast carcinoma 1 centimeter or less in size (T1a, bNOMO): clinicopathologic features and outcome. Cancer. 1997;79(4):761–71.
Parise CA, Caggiano V. Risk of mortality of node-negative, ER/PR/HER2 breast cancer subtypes in T1, T2, and T3 tumors. Breast Cancer Res Treat. 2017;165(3):743–50.
Cole BF, Gelber RD, Gelber S, Coates AS, Goldhirsch A. Polychemotherapy for early breast cancer: an overview of the randomised clinical trials with quality-adjusted survival analysis. Lancet. 2001;358(9278):277–86.
Tao JJ, Visvanathan K, Wolff AC. Long term side effects of adjuvant chemotherapy in patients with early breast cancer. Breast. 2015;24 Suppl 2(0 2):S149-153.
Ravdin PM, Siminoff IA, Harvey JA. Survey of breast cancer patients concerning their knowledge and expectations of adjuvant therapy. J Clin Oncol. 1998;16(2):515–21.
Berry DA, Cronin KA, Plevritis SK, Fryback DG, Clarke L, Zelen M, Mandelblatt JS, Yakovlev AY, Habbema JD, Feuer EJ. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353(17):1784–92.
Kowal P, Dowd J. Definition of an older person. Proposed working definition of an older person in Africa for the MDS Project. 2001.
Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150–61.
Kennedy T, Stewart AK, Bilimoria KY, Patel-Parekh L, Sener SF, Winchester DP. Treatment trends and factors associated with survival in T1aN0 and T1bN0 breast cancer patients. Ann Surg Oncol. 2007;14(10):2918–27.
Von Hoff DD, Layard MW, Basa P, Davis HL Jr, Von Hoff AL, Rozencweig M, Muggia FM. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91(5):710–7.
Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T, Wolff AC, Sledge GW Jr, Wood WC, Davidson NE. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358(16):1663–71.
Smith RE. Risk for the development of treatment-related acute myelocytic leukemia and myelodysplastic syndrome among patients with breast cancer: review of the literature and the National Surgical Adjuvant Breast and Bowel Project experience. Clin Breast Cancer. 2003;4(4):273–9.
Petrek JA, Naughton MJ, Case LD, Paskett ED, Naftalis EZ, Singletary SE, Sukumvanich P. Incidence, time course, and determinants of menstrual bleeding after breast cancer treatment: a prospective study. J Clin Oncol. 2006;24(7):1045–51.
Hanrahan EO, Gonzalez-Angulo AM, Giordano SH, Rouzier R, Broglio KR, Hortobagyi GN, Valero V. Overall survival and cause-specific mortality of patients with stage T1a, bN0M0 breast carcinoma. J Clin Oncol. 2007;25(31):4952–60.
Montero A, Ciérvide R, García-Aranda M, Rubio C. Postmastectomy radiation therapy in early breast cancer: Utility or futility? Crit Rev Oncol Hematol. 2020;147:102887.
Wang K, ** X, Wang W, Yu X, Huang J. The role of postmastectomy radiation in patients with ypN0 breast cancer after neoadjuvant chemotherapy: a meta-analysis. BMC Cancer. 2021;21(1):728.
Wu S, **n Z, Sui D, Ou Z, Bai H, Zhu S, Wang X, Zhang J. Development and validation of a nomogram to predict drainage duration in patients with breast cancer treated with modified radical mastectomy. Sci Rep. 2021;11(1):2533.
Liljegren G, Holmberg L, Bergh J, Lindgren A, Tabár L, Nordgren H, Adami HO. 10-Year results after sector resection with or without postoperative radiotherapy for stage I breast cancer: a randomized trial. J Clin Oncol. 1999;17(8):2326–33.
Fisher B, Bryant J, Dignam JJ, Wickerham DL, Mamounas EP, Fisher ER, Margolese RG, Nesbitt L, Paik S, Pisansky TM, et al. Tamoxifen, radiation therapy, or both for prevention of ipsilateral breast tumor recurrence after lumpectomy in women with invasive breast cancers of one centimeter or less. J Clin Oncol. 2002;20(20):4141–9.
Winzer KJ, Sauerbrei W, Braun M, Liersch T, Dunst J, Guski H, Schumacher M. Radiation therapy and tamoxifen after breast-conserving surgery: updated results of a 2 x 2 randomised clinical trial in patients with low risk of recurrence. Eur J Cancer. 2010;46(1):95–101.
Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, Godwin J, Gray R, Hicks C, James S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–106.
Hejazi E, Nasrollahzadeh J, Fatemi R, Barzegar-YarMohamadi L, Saliminejad K, Amiri Z, Kimiagar M, Houshyari M, Tavakoli M, Idali F. Effects of Combined Soy Isoflavone Extract and Docetaxel Treatment on Murine 4T1 Breast Tumor Model. Avicenna J Med Biotechnol. 2015;7(1):16–21.
Hajian S, Mehrabi E, Simbar M, Houshyari M, Zayeri F, Hajian P. Designing and Psychometric Evaluation of Adjustment to Illness Measurement Inventory for Iranian Women With Breast Cancer. Iran J Cancer Prev. 2016;9(4):e5461.
Servick K. Breast cancer. Breast cancer: a world of differences. Science. 2014;343(6178):1452–3.
National Comprehensive Cancer Network (2019) NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Breast Cancer Version 1. 2019. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed 21 Feb 2020.
Curigliano G, Burstein HJ, Winer EP, Gnant M, Dubsky P, Loibl S, Colleoni M, Regan MM, Piccart-Gebhart M, Senn HJ, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann Oncol. 2017;28(8):1700–12.
Goldstein LJ, Gray R, Badve S, Childs BH, Yoshizawa C, Rowley S, Shak S, Baehner FL, Ravdin PM, Davidson NE, et al. Prognostic utility of the 21-gene assay in hormone receptor-positive operable breast cancer compared with classical clinicopathologic features. J Clin Oncol. 2008;26(25):4063–71.
Mamounas EP, Tang G, Fisher B, Paik S, Shak S, Costantino JP, Watson D, Geyer CE Jr, Wickerham DL, Wolmark N. Association between the 21-gene recurrence score assay and risk of locoregional recurrence in node-negative, estrogen receptor-positive breast cancer: results from NSABP B-14 and NSABP B-20. J Clin Oncol. 2010;28(10):1677–83.
Sparano JA, Paik S. Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol. 2008;26(5):721–8.
Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Perez EA, Olson JA Jr, et al. Prospective Validation of a 21-Gene Expression Assay in Breast Cancer. N Engl J Med. 2015;373(21):2005–14.
Gluz O, Nitz UA, Christgen M, Kates RE, Shak S, Clemens M, Kraemer S, Aktas B, Kuemmel S, Reimer T, et al. West German Study Group Phase III PlanB Trial: First Prospective Outcome Data for the 21-Gene Recurrence Score Assay and Concordance of Prognostic Markers by Central and Local Pathology Assessment. J Clin Oncol. 2016;34(20):2341–9.
Cardoso F, van’t Veer LJ, Bogaerts J, Slaets L, Viale G, Delaloge S, Pierga JY, Brain E, Causeret S, DeLorenzi M, et al. 70-Gene Signature as an Aid to Treatment Decisions in Early-Stage Breast Cancer. N Engl J Med. 2016;375(8):717–29.
Tsukamoto F, Arihiro K, Takahashi M, Ito KI, Ohsumi S, Takashima S, Oba T, Yoshida M, Kishi K, Yamagishi K, et al. Multicenter retrospective study on the use of Curebest™ 95GC Breast for estrogen receptor-positive and node-negative early breast cancer. BMC Cancer. 2021;21(1):1077.
Senkus E, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rutgers E, Zackrisson S, Cardoso F. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v8-30.
An X, Lei X, Huang R, Luo R, Li H, Xu F, Yuan Z, Wang S, de Nonneville A, Gonçalves A, et al. Adjuvant chemotherapy for small, lymph node-negative, triple-negative breast cancer: A single-center study and a meta-analysis of the published literature. Cancer. 2020;126(Suppl 16):3837–46.
Steenbruggen TG, van Werkhoven E, van Ramshorst MS, Dezentjé VO, Kok M, Linn SC, Siesling S, Sonke GS. Adjuvant chemotherapy in small node-negative triple-negative breast cancer. Eur J Cancer. 2020;135:66–74.
Gao ZH, Li CX, Liu M, Jiang JY. Predictive and prognostic role of tumour-infiltrating lymphocytes in breast cancer patients with different molecular subtypes: a meta-analysis. BMC Cancer. 2020;20(1):1150.
Guo L, **e G, Wang R, Yang L, Sun L, Xu M, Yang W, Chung MC. Local treatment for triple-negative breast cancer patients undergoing chemotherapy: breast-conserving surgery or total mastectomy? BMC Cancer. 2021;21(1):717.
Bhoo-Pathy NT, Inaida S, Tanaka S, Taib NA, Yip CH, Saad M, Kawakami K, Bhoo-Pathy N. Impact of adjuvant chemotherapy on survival of women with T1N0M0, hormone receptor negative breast cancer. Cancer Epidemiol. 2017;48:56–61.
Sonnenblick A, Fumagalli D, Azim HA Jr, Sotiriou C, Piccart M. New strategies in breast cancer: the significance of molecular subtypes in systemic adjuvant treatment for small T1a, bN0M0 tumors. Clin Cancer Res. 2014;20(24):6242–6.
Hanrahan EO, Valero V, Gonzalez-Angulo AM, Hortobagyi GN. Prognosis and management of patients with node-negative invasive breast carcinoma that is 1 cm or smaller in size (stage 1; T1a, bN0M0): a review of the literature. J Clin Oncol. 2006;24(13):2113–22.
Raffaello W, Kurniawan A. Sarcopenic Obesity in Cancer Patients: Focus on Pathogenesis. Indonesian J Cancer. 2020;14:104–14.
Ginzac A, Barres B, Chanchou M, Gadéa E, Molnar I, Merlin C, Coudert B, Thivat E, Durando X. A decrease in brown adipose tissue activity is associated with weight gain during chemotherapy in early breast cancer patients. BMC Cancer. 2020;20(1):96.
Acknowledgements
The authors thank the data managers and staff of the Surveillance, Epidemiology, and End Results (SEER) database for the collection of data in the USA Cancer Registry. We would like to thank the patients from Northern Jiangsu People’s Hospital for the clinical data.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
The first 4 authors contributed equally to this article. Kaiwen Shen: Writing–original draft and formal analysis. Longdi Yao, **gyuan Zhu, **ming Gu: Writing–review and editing. Jie Wang, Wei Qian: Data acquisition and editing. Zhijian Zheng: Figure editing and typesetting. Deyuan Fu: Data acquisition and data analysis. Song Wu: Study design and data analysis. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The SEER Program collects data from population‐based cancer registries with anonymous information. The SEER is a publicly available database and data extracted from SEER was deemed “non‐human study” by the North Shore LIJ IRB committee.
This article is a retrospective review of patient data, and the study design was approved by the Medical Ethics Committee of Northern Jiangsu People’s Hospital. This article does not contain any studies with animals or human participants performed by any of the authors, and all methods were carried out in accordance with the Declaration of Helsinki.
For this type of study, formal consent is not required, and the need for informed consent was waived by the Medical Ethics Committee of Northern Jiangsu People’s Hospital.
Consent for publication
Not applicable.
Competing interests
The authors have no conflict of interest to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Figure S1.
Kaplan–Meier survival curves of the chemotherapy and nochemotherapy groups according to grades and molecular subtypes of T1a breastcancer patients treated at Northern Jiangsu People’s Hospital. (A) Grade I; (B)grade II; (C) HoR+/HER2-; (D) HoR+/HER2+; and (E) HoR-/HER2+. Abbreviations: HoR: hormonereceptor; HER2: human epidermal growth factor receptor‐2.
Additional file 2: Figure S2.
Kaplan–Meier survival curves of the chemotherapy and nochemotherapy groups according to the grades and molecular subtypes of T1bbreast cancer patients treated at Northern Jiangsu People’s Hospital. (A) GradeI; (B) grade II; (C) grade III; (D) HoR+/HER2-; (E) HoR+/HER2+; (F) HoR-/HER2+;and (G) HoR-/HER2-. Abbreviations: HoR: hormone receptor; HER2: human epidermal growthfactor receptor‐2.
Additional file 3: Figure S3.
Kaplan–Meier survival curves of the chemotherapy and nochemotherapy groups according to the grades and molecular subtypes of T1cbreast cancer patients treated at Northern Jiangsu People’s Hospital. (A) GradeI; (B): grade II; (C) grade III; (D) HoR+/HER2-; (E) HoR+/HER2+; (F) HoR-/HER2+;and (G) HoR-/HER2-. Abbreviations: HoR: hormone receptor; HER2: human epidermal growthfactor receptor‐2.
Additional file 4: Table S1.
Demographic andclinical characteristics of the included T1N0M0 breast cancer patients in theSEER database.
Additional file 5: Table S2.
Multivariate Cox regressionanalyses of overall survival for T1, T1a, T1b, and T1c breast cancer patients.
Additional file 6: Table S3.
Multivariate Cox regressionanalyses of overall survival for T1, T1a, T1b, and T1c breast cancer patientsregarding treatment methods.
Additionalfile 7: Table S4.
MultivariableCox regression analyses of overall survival forfour molecular subtypes in T1a breast cancer patients.
Additional file 8: Table S5.
Multivariable Cox regression analyses of overall survival for tumor grades in T1a breast cancer patients.
Additional file 9: Table S6.
Multivariable Cox regression analyses of overall survival for molecularsubtypes in T1b breast cancer patients.
Additional file 10: Table S7.
Multivariable Cox regression analyses of overall survival for tumorgrades in T1b breast cancer patients.
Additional file 11: Table S8.
Multivariable Coxregression analyses of overall survival for molecular subtypes in T1c breast cancer patients.
Additional file 12: Table S9.
Multivariable Coxregression analyses of overall survival for tumor grades in T1c breast cancerpatients.
Additional file 13: Table S10.
Multivariable Coxregression analyses of overall survival for tumor grades in HoR+/HER2- T1abreast cancer patients.
Additional file 14: Table S11.
Multivariable Cox regression analyses of overall survival for tumorgrades in HoR+/HER2+ T1abreast cancer patients.
Additional file 15: Table S12.
Multivariable Cox regression analyses of overall survival for tumorgrades in HoR-/HER2+ T1a breast cancer patients.
Additional file 16: Table S13.
Multivariable Cox regression analyses of overall survival for tumorgrades in HoR-/HER2- T1a breast cancer patients.
Additional file 17: Table S14.
Multivariable Coxregression analyses of overall survival for tumor grades in HoR+/HER2- T1b breastcancer patients.
Additional file 18: Table S15.
Multivariable Coxregression analyses of overall survival for tumor grades in HoR+/HER2+ T1b breast cancer patients.
Additional file 19: Table S16.
Multivariable Coxregression analyses of overall survival for tumor grades in HoR-/HER2+ T1b breast cancer patients.
Additional file 20: Table S17.
MultivariableCox regression analysesof overall survival for tumor grades in HoR-/HER2- T1b breast cancer patients.
Additional file 21: Table S18.
Multivariable Cox regression analyses of overall survival for tumorgrades in HoR+/HER2- T1c breast cancer patients.
Additional file 22: Table S19.
Multivariable Cox regression analyses of overall survival fortumor grades in HoR+/HER2+ T1c breast cancer patients.
Additional file 23: Table S20.
Multivariable Cox regression analyses of overall survival for tumorgrades in HoR-/HER2+ T1c breast cancer patients.
Additional file 24: Table S21.
Multivariable Cox regression analysesof overall survival for tumor grades in HoR-/HER2- T1c breast cancer patients.
Additional file 25: Table S22.
Demographic and clinicalcharacteristics of the included T1N0M0 breast cancer patients in Northern Jiangsu People’sHospital.
Additional file 26: Table S23.
Univariableand multivariable Cox regression analyses of overallsurvival for T1a breast cancer patients in Northern Jiangsu People’s Hospital.
Additional file 27: Table S24.
Univariable and multivariable Cox regression analyses ofoverall survival for T1b breast cancer patients in Northern Jiangsu People’s Hospital.
Additional file 28: Table S25.
Univariable and multivariable Cox regression analyses ofoverall survival for T1c breast cancer patients in Northern Jiangsu People’sHospital.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shen, K., Yao, L., Zhu, J. et al. Impact of adjuvant chemotherapy on T1N0M0 breast cancer patients: a propensity score matching study based on SEER database and external cohort. BMC Cancer 22, 863 (2022). https://doi.org/10.1186/s12885-022-09952-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12885-022-09952-z