Abstract
Background
Despite many guidelines for the management of gestational diabetes available internationally, little work has been done to summarize and assess the content of existing guidelines. A paucity of analysis guidelines within in a unified system may be one explanatory factor. So this study aims to analyze and evaluate the contents of all available guidelines for the management of gestational diabetes.
Method
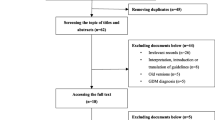
Relevant clinical guidelines were collected through a search of relevant guideline websites and databases (PubMed, Web of Science, Embase, etc.). Fourteen guidelines were identified, and each guideline was assessed for quality using the Appraisal of Guidelines for Research & Evaluation (AGREE) II instrument. Two independent reviewers extracted guideline recommendations using a “recommendation matrix” through which basic guideline information and consistency between search strategy and selection of evidence, between selected evidence and interpretation, and between interpretation and resulting recommendations were analyzed.
Results
Fourteen documents were analyzed, and a total of 361 original recommendations for gestational diabetes mellitus (GDM) management were assessed. In all guidelines included, the recommendations were developed in five domains, namely, diagnosis of GDM, prenatal care, intrapartum care, neonatal care and postpartum care. Different guidelines appeared to have significant discrepancy in consistency of guideline content, but overall, there was consistency between search strategy and selection of evidence, between selected evidence and interpretation, and between interpretation and resulting recommendations (scilicet 49.31, 57.20 and 58.17%, respectively).
Conclusion
Although commonality in most recommendations existed, there were still some discrepancies between guidelines. Consistency of guidelines on the management of GDM in pregnancy is highly variable and needs to be improved.
Similar content being viewed by others
Background
Gestational diabetes mellitus (GDM) is a special form of diabetes in women of child-bearing age and is a common gestational endocrine disease [1]. Due to its increasing prevalence, GDM results in significant short- and long-term impairments in the individual’s health and their offspring’s health [Assessment of consistency A total of 361 original recommendations for GDM management which were from 14 guidelines were included. Although some recommendations did not fall into any of the identified themes, we undertook consistency appraisal of these as well. As presented in Table 5, different guidelines appeared to have significant discrepancies in consistency of guideline content. Even in the same guideline, consistency differed in three aspects: ①consistency between search strategy and selection of evidence, ②consistency between selected evidence and interpretation, and ③consistency between interpretations and resulting recommendations. Among all guidelines included, NICE guidelines showed the best average score of consistency in each aspect. However, HKCOG guidelines and CMA guidelines received extremely low scores in each aspect. Apparently, in this study, evidence-based guidelines rated relatively higher in content consistency than expert consensus-based guidelines. Consistency appraisal of each guideline is presented in Fig. 2. For consistency in each aspect, most guidelines showed the same tendency, that is, a guideline which received high average scores could also receive high scores in the other two aspects, and, conversely, low average scores in all aspects. When it came to all recommendations, search strategy and selection of evidence were slightly inconsistent. The radar chart showing comparable consistency between search strategy and selection of evidence, between selected evidence and interpretation, and between interpretation and resulting recommendations (scilicet 49.31, 57.20 and 58.17%, respectively) is presented in Fig. 3.
Discussion
Gestational diabetes mellitus is a challenging complication of pregnancy that many women and doctors struggle with. In this review, we examined the existing guidelines on the management for GDM in 11 countries or regions. Given that appropriate methodologies and rigorous strategies in the guideline development process are crucial for guideline implementation [25], the development methods of the guidelines were measured using the AGREE II instrument. In general, the quality of GDM guidelines, especially evidence-based guidelines, was high. This could be explained by the fact that much progress has been made in the development of methodological and reporting criteria of evidence-based guidelines within the past decade [30]. Nonetheless, as the results in previous study revealed, the domains of Rigor of Development, Stakeholder Involvement and, Editorial Independence still need to be improved [26].
It is noted that practice guidelines with the best methodological quality were not necessarily the most valid in their recommendations [27]. Thus it is important to emphasize that clinical practitioners should critically evaluate the methodological quality as well as the content of the recommendations before adopting the recommendations, which leads to another issue, that is, consistency appraisal. Despite many researchers being aware of the crucial role of the appraisal of consistency between evidence and resulting recommendations, there are no existing criteria for assessing content consistency of guidelines. In guideline adaptation of some topics, qualitative analysis was used in content extraction, which formulated a general description of the research topic through generating categories without any consistency appraisal [31, 32]. In this review, we developed a “recommendation matrix” on the basis of the CAN-Implement© method [33], and used the tool to extract and assess guideline content. As a recommendation matrix was used, not only relevant and potentially relevant recommendations on all pre-specified healthcare aspects for GDM care were identified, but also consistency between search strategy and selection of evidence, between selected evidence and interpretation, and between interpretation and resulting recommendations was assessed. The results showed that current guidelines on GDM care are of varied consistency, and guidelines developed in internationally recognized guideline development methodology show better consistency. Also guidelines that have low consistency in one aspect may also have low consistency in other two aspects. This is probably because reporting quality of guidelines is the cornerstone of consistency assessment. Those guidelines with evidence tables or technical reports not published may also show low consistency. Thus, guideline development committees are strongly encouraged to make use of guideline development manuals when drafting guidelines.
Regarding guideline content, five aspects were analyzed: diagnosis of GDM, prenatal care, intrapartum care, neonatal care, and postpartum care. Most recommendations in guidelines focused on prenatal care, especially all kinds of therapies that might reduce the risk of adverse pregnancy outcomes related to uncontrolled blood sugar pre-conception. This review generated similar results with those from a previous study that international guidelines were consistent in most of their recommendations [34]. Nonetheless, although commonality in most areas existed, there were still some discrepancies among guidelines. For example, recommendations regarding oral hypoglycemic agents in the guidelines diverged. Some guidelines recommended that oral hypoglycemic agents be considered as an initial pharmacological intervention, while some guidelines only considered insulin as an exclusive hypoglycemic medicine. Guidelines were supported with evidence, so inconsistency may be caused by insufficient evidence on pharmacological interventions in the period in which the guidelines were developed [26]. However, it should be reminded that even though all evidence available was identified, consensus usually did not warrant similar recommendations in different contexts. This was because when a recommendation was developed, not only available evidence, but benefits and harms, patients’ values and preferences, as well as resource implications, should be appropriate considered [10].
Since recommendations were well summarized, guideline adaptation was required to maintain the validity of recommendations in different health care systems. Guideline adaptation involves using knowledge synthesis of existing guidelines to produce recommendations, rather than relying only on a review of primary literature, for the purpose of reducing duplication of effort [35]. In mainland China and Hong Kong, there were only expert consensus for GDM care [22, 23], without a national GDM management evidence-based guideline adapted to the Chinese context previously. In this instance, it is recommended to adapt the clinical practice guideline related to GDM management for the local context, providing support for professionals to make better decisions in clinical practice. How to select, tailor and implement recommendations and supporting evidence extracted is the next challenging step.
Limitation
Due to the language barriers, we only included guidelines in English and Chinese. As a result, we only got existing guidelines on the management for GDM in 11 countries or regions in this review. And yet, we have no idea whether other countries use the recommendations provided by a certain guideline or use recommendations developed in their own language.
Another key limitation of this study is the subjectivity in appraising the consistency between evidence and recommendations. Although we attempted to minimize these discrepancies by stating the assessment criteria and through rigorous discussion, the results of the consistency appraisal still varied because of different understandings between researchers. Additionally, reporting quality of some guidelines is not clear cut, which was another barrier in the process of content analysis. Apart from this, this is the first time that we used a “recommendation matrix” in content analysis, and the tool we developed may still need to be modified.
Conclusion
This paper describes the process used to extract and access the content of guidelines for GDM management. In conclusion, the recommendations were developed in five aspects: diagnosis of GDM, prenatal care, intrapartum care, neonatal care and postpartum care. The consistency of guidelines on the management of GDM in pregnancy is highly variable and this inconsistency needs to be addressed. Also, this review has proven that a “recommendation matrix” can be a tool to extract and assess consistency of guidelines. Additionally, our findings indicated that it is necessary to adapt and disseminate easily understandable evidence-based guidelines based on knowledge synthesis of existing guidelines in this paper.
Abbreviations
- A.N.D:
-
Academy of Nutrition and Dietetics
- ADA:
-
American Diabetes Association
- API:
-
The Association of Physicians of India
- CDA:
-
Canadian Diabetes Association
- CMA:
-
Chinese Medical Association
- DDG:
-
German Diabetes Association
- DGGG:
-
German Gynecology and Obstetrics Association
- FIGO:
-
The International Federation of Gynecology and Obstetrics
- HKCOG:
-
The Hong Kong College of Obstetricians and Gynaecologists
- IDF:
-
International Diabetes Federation
- NCC-WCH:
-
National Collaborating Centre for Women's and Children's Health
- NGC:
-
National Guideline Clearinghouse
- NICE:
-
The National Institute for Health and Care Excellence
- NZGG:
-
New Zealand Guidelines Group
- SIGN:
-
Scottish Intercollegiate Guidelines Network
- WHO:
-
World Health Organization
References
World Health Organization. Diagnostic criteria and classification of Hyperglycaemia first detected in pregnancy. Geneva: World Health Organization; 2013.
Wu L, Han L, Zhan Y, Cui L, Chen W, Ma L, Lv J, Pan R, Zhao D, **ao Z. Prevalence of gestational diabetes mellitus and associated risk factors in pregnant Chinese women: a cross-sectional study in Huangdao, Qingdao, China. Asia Pac J Clin Nutr. 2018;27(2):383–8.
Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, Lowe LP, Trimble ER, Coustan DR, Hadden DR, et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care. 2012;35(4):780–6.
Ovesen PG, Jensen DM, Damm P, Rasmussen S, Kesmodel US. Maternal and neonatal outcomes in pregnancies complicated by gestational diabetes. A nation-wide study. J Matern Fetal Neonatal Med. 2015;28(14):1720–4.
Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115(3):e290–6.
West NA, Crume TL, Maligie MA, Dabelea D. Cardiovascular risk factors in children exposed to maternal diabetes in utero. Diabetologia. 2011;54(3):504–7.
Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Donovan L. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S. preventive services task force and the National Institutes of Health Office of medical applications of research. Ann Intern Med. 2013;159(2):123–9.
Nisreen A, Derek JT, Jane W. Treatments for gestational diabetes. Cochrane Database Syst Rev. 2009;(3). https://doi.org/10.1002/14651858.CD003395.pub2.
Feng R, Liu L, Zhang YY, Yuan ZS, Gao L, Zuo CT. Unsatisfactory glucose management and adverse pregnancy outcomes of gestational diabetes mellitus in the real world of clinical practice: a retrospective study. Chin Med J. 2018;131(9):1079–85.
World Health Organization: WHO handbook for guideline development. 2012. Accessed 2018.
Academy Of Nutrition And Dietetics. Academy of nutrition and dietetics gestational diabetes mellitus evidence-based nutrition practice guideline. Chicago: Academy of Nutrition and Dietetics; 2016.
Canadian Diabetes Association Clinical Practice Guidelines Expert Committee. Clinical practice guidelines: diabetes and pregnancy. Can J Diabetes. 2013;37:S168–83.
Endocrine Society. Diabetes and pregnancy: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98:4227–49.
IDF Clinical Guidelines Task Force. Global Guideline on Pregnancy and Diabetes. Brussels: International Diabetes Federation; 2009.
Ministry Of Health. Screening, Diagnosis and Management of Gestational Diabetes in New Zealand: A clinical practice guideline. Wellington: Ministry of Health; 2014.
Queensland Health. Queensland Clinical Guideline: Gestational diabetes mellitus Guideline. Queensland: Queensland Health; 2015. Accessed 2018.
Scottish Intercollegiate Guidelines Network: Managemenet of diabetes: a national clinical guideline. 2013. Accessed 2018.
The International Federation of Gynecology and Obstetrics (FIGO). Initiative on gestational diabetes mellitus: a pragmatic guide for diagnosis, management, and care. Int J Gynecol Obstet. 2015;131(S3):S173–211.
Seshiah V, Banerjee S, Balaji V, Muruganathan A, Das AK. Consensus evidence-based guidelines for management of gestational diabetes mellitus in India. J Assoc Physicians India. 2014;62(7 Suppl):55–62.
American Diabetes Association. Standards of medical Care in Diabetes—2018. Diabetes Care. 2018;41(suppl.1):s1–s159.
National Collaborating Centre for Women's and Children's Health: Diabetes in pregnancy: Management of diabetes and its complications from preconception to the postnatal period. 2015.Accessed 2018.
The Hong Kong College of Obstetricians and Gynaecologists. Guidelines for the Management of Gestational Diabetes Mellitus. 2016. Accessed in http://www.hkcog.org.hk/hkcog/Download/Guidelines_on_GDM_updated.pdf.
The Chinese medical association branch of obstetrics and gynaecology obstetric group. Diagnosis and management of diabetes in pregnancy: a clinical practice guideline (2014). Chin J Obstet Gynecol. 2014;49(8):561–9.
Woolf SH, Grol R, Hutchinson A, Eccles M, Grimshaw J. Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ. 1999;318(7182):527–30.
Brouwers MC, Kho ME, Browman GP, Cluzeau F, feder G, Fervers B, Hanna S, Makarski J. AGREE II: advancing guideline development, reporting and evaluation in healthcare. Can Med Assoc J. 2010;182(18):E839–42.
Greuter MJ, van Emmerik NM, Wouters MG, van Tulder MW. Quality of guidelines on the management of diabetes in pregnancy: a systematic review. BMC Pregnancy Childbirth. 2012;12:58.
Watine J, Friedberg B, Nagy E, Onody R, Oosterhuis W, Bunting PS, Charet JC, Horvath AR. Conflict between guideline methodologic quality and recommendation validity: a potential problem for practitioners. Clin Chem. 2006;52(1):65–72.
The ADAPTE Collaboration: Guideline adaption: a resource toolkit. 2010. Accessed 2018.
Schafer-Graf UM, Gembruch U, Kainer F, Groten T, Hummel S, Hosli I, Grieshop M, Kaltheuner M, Buhrer C, Kautzky-Willer A, et al. Gestational diabetes mellitus (GDM) - diagnosis, treatment and follow-up. Guideline of the DDG and DGGG (S3 level, AWMF registry number 057/008, February 2018). Geburtshilfe Frauenheilkd. 2018;78(12):1219–31.
Wang Y, ** Y, Chen Y, Ceng X, Chen H, Wang L, Lu C, Cao H. The methodology of recommendations in evidence-based clinical practice guideline. Chin J Evid Based Med. 2017;09:1085–92.
Hu Y, Cheng Y. Content analysis of existing clinical practice guidelines related to nasogastric feeding in adult patients. Chin J Nurs. 2014;49(10):1177–83.
Yu L, Hu J, Hao G, Ruan H. Content analysis of international clinical practice guidelines related to endotracheal suctioning of adults with an Artifcial airway. West Chin Med J. 2014;29(10):1922–6.
Harrison MB, van den Hoek J. CAN-IMPLEMENT: Guideline Adaptation and Implementation Planning Resource; 2012.
Mahmud M, Mazza D. Preconception care of women with diabetes: a review of current guideline recommendations. BMC Womens Health. 2010;10:5.
Selby P, Hunter K, Rogers J, Lang-Robertson K, Soklaridis S, Chow V, Tremblay M, Koubanioudakis D, Dragonetti R, Hussain S, et al. How to adapt existing evidence-based clinical practice guidelines: a case example with smoking cessation guidelines in Canada. BMJ Open. 2017;7(11):e16124.
Acknowledgements
We would like to thank Siew Siang Tay for hel** us to revise the final version of the article.
Funding
This study was undertaken under grant 2016 Project of Shanghai Municipal Commission of Health and family Planning (No:201640324), and grant 2015 Nursing Research Project of Fudan University (No:FNF201502). The funders had no involvement in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
Availability of data and materials
All data analyzed during this study are included in this published article.
Author information
Authors and Affiliations
Contributions
All authors have made substantial contributions and gave final approval of the conceptions, drafting, and final version of this manuscript. The authors’ contributions are presented by their initials. MZ contributed to the data collection, review of the guidelines, and content extraction and the writing of this manuscript. YZ contributed to the data collection, review of the guidelines, and content extraction and the writing of this manuscript. JZ participated in the review of the guidelines, and helped to translate the recommendations. She also participated in the interpretation of data. KW participated in the review and translation of the guidelines. LL and YD participated in the recommendation appraisal, and helped to develop the “recommendation matrix” tool. All authors have seen and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declared that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Recommendations Extraction of NICE guideline. The NICE guideline was developed in accordance with the NICE guideline development process. There were 74 relevant recommendations being extracted, which were displayed and appraised in Additional file 1. (XLSX 24 kb)
Additional file 2:
Recommendations Extraction of NZGG guideline. The NZGG guideline development team followed a structured process for guideline development, and there were 38 relevant recommendations being extracted, which were displayed and appraised in Additional file 2. (XLSX 19 kb)
Additional file 3:
Recommendations Extraction of SIGN guideline. The SIGN guideline was developed using a standard methodology according to SIGN guideline manual. There were 18 relevant recommendations being extracted, which were displayed and appraised in Additional file 3. (XLSX 16 kb)
Additional file 4:
Recommendations Extraction of ADA guideline. The ADA guideline was developed using a standard methodology by the ADA’s Professional Practice Committee. The guideline included general recommendations about all kinds of diabetes, and there were only 17 relevant recommendations being extracted, which were displayed and appraised in Additional file 4. (XLSX 15 kb)
Additional file 5:
Recommendations Extraction of FIGO guideline. The FIGO brought together international experts to develop the guideline, and suggestions are provided for a variety of different regional and resource settings. There were 40 relevant recommendations being extracted, which were displayed and appraised in Additional file 5. (XLSX 19 kb)
Additional File 6:
Recommendations Extraction of Endocrine Society guideline. The Endocrine Society guideline was searched on the NGC website, which provided recommendations for the management of the pregnant woman with diabetes. Twenty-five relevant recommendations were extracted and appraised, which were displayed in Additional file 6. (XLSX 16 kb)
Additional file 7:
Recommendations Extraction of CDA guideline. The CDA guideline was developed following the process used to develop previous Canadian Diabetes Association clinical practice guidelines, and AGREE II were incorporated into the guideline development process. There were 17 relevant recommendations being extracted, which were displayed and appraised in Additional file 7. (XLSX 15 kb)
Additional file 8:
Recommendations Extraction of API guideline. To develop API guideline, existing guidelines, meta-analyses, cross sectional studies, systematic reviews and key cited articles were reviewed, and the recommendations were discussed at the national insulin summit. There were 22 relevant recommendations being extracted, which were displayed and appraised in Additional file 8. (XLSX 17 kb)
Additional file 9:
Recommendations Extraction of IDF guideline. The guideline was developed through a non-formal evidence review and discussed by a small Writing Group. There were 13 relevant recommendations being extracted, which were displayed and appraised in Additional file 9. (XLSX 14 kb)
Additional file 10:
Recommendations Extraction of Queensland guideline. The Queensland guideline was developed based on evidence, and there were 8 relevant recommendations being extracted, which were displayed and appraised in Additional file 10. (XLSX 14 kb)
Additional file 11:
Recommendations Extraction of HKCOG guideline. The HKCOG guideline was an expert consensus. It was updated taking reference to the recent evidence, WHO, NICE guideline and recommendations of other international bodies. There were 13 relevant recommendations being extracted, which were displayed and appraised in Additional file 11. (XLSX 14 kb)
Additional file 12:
Recommendations Extraction of A.N.D. guideline. The guideline focused on nutrition practiece during the treatment of women with GDM. There were 15 relevant recommendations being extracted, which were displayed and appraised in Additional file 12. (XLSX 15 kb)
Additional file 13:
Recommendations Extraction of DDG guideline. The recommendations of DDG guideline were based on the evidence from the literature, which was selected through a systematic external literature search. There were 21 relevant recommendations being extracted, which were displayed and appraised in Additional file 13. (XLSX 15 kb)
Additional file 14:
Recommendations Extraction of CMA guideline. The CMA guideline was an expert consensus. There were 40 relevant recommendations being extracted, which were displayed and appraised in Additional file 14. (XLSX 19 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhang, M., Zhou, Y., Zhong, J. et al. Current guidelines on the management of gestational diabetes mellitus: a content analysis and appraisal. BMC Pregnancy Childbirth 19, 200 (2019). https://doi.org/10.1186/s12884-019-2343-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12884-019-2343-2