Abstract
Background
Delayed graft function (DGF) is an important complication after kidney transplantation surgery. The present study aimed to develop and validate a nomogram for preoperative prediction of DGF on the basis of clinical and histological risk factors.
Methods
The prediction model was constructed in a development cohort comprising 492 kidney transplant recipients from May 2018 to December 2019. Data regarding donor and recipient characteristics, pre-transplantation biopsy results, and machine perfusion parameters were collected, and univariate analysis was performed. The least absolute shrinkage and selection operator regression model was used for variable selection. The prediction model was developed by multivariate logistic regression analysis and presented as a nomogram. An external validation cohort comprising 105 transplantation cases from January 2020 to April 2020 was included in the analysis.
Results
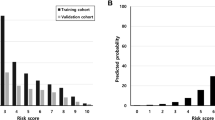
266 donors were included in the development cohort, 458 kidneys (93.1%) were preserved by hypothermic machine perfusion (HMP), 96 (19.51%) of 492 recipients developed DGF. Twenty-eight variables measured before transplantation surgery were included in the LASSO regression model. The nomogram consisted of 12 variables from donor characteristics, pre-transplantation biopsy results and machine perfusion parameters. Internal and external validation showed good discrimination and calibration of the nomogram, with Area Under Curve (AUC) 0.83 (95%CI, 0.78–0.88) and 0.87 (95%CI, 0.80–0.94). Decision curve analysis demonstrated that the nomogram was clinically useful.
Conclusion
A DGF predicting nomogram was developed that incorporated donor characteristics, pre-transplantation biopsy results, and machine perfusion parameters. This nomogram can be conveniently used for preoperative individualized prediction of DGF in kidney transplant recipients.
Similar content being viewed by others
Introduction
Delayed graft function (DGF) is a common complication in organ transplantation. DGF is a form of acute renal failure [1] that can result in increased allograft immunogenicity, leading to subsequent acute rejection and graft failure. As reported earlier [2], DGF is remarkably associated with long-term dysfunction. There is no consensus in the literature about how to define DGF. The straightforward United Network for Organ Sharing definition of DGF is the need for at least one dialysis treatment in the first week after transplantation (classical DGF) [3]. According to the 2018 Annual Report of the Organ Procurement and Transplantation Network/Scientific Registry of Transplant Recipients (OPTN/SRTR) [4] and the 2016 Annual Report of the European Renal Association-European Dialysis and Transplant Association (ERA-EDTA) Registry [5], the incidence of DGF ranged between 20% and 30% in the United States and almost 50% in Europe.
DGF can lead to several health-related consequences. It not only increases the risk of graft failure but also prolongs hospitalization, thereby increasing healthcare-related expenditure. Additionally, a high rate of mortality is reported in recipients diagnosed to have DGF [6].
DGF is a multifactorial event. The risk factors of DGF include donor and recipient characteristics, pre-transplantation biopsy results [7], and machine perfusion parameters [8]. Irish et al. developed a primary DGF prediction model based on a nomogram by considering donor and recipient clinical factors alone [9]. The incorporation of machine perfusion parameters and pre-transplantation biopsy results into a clinical variable-based predictive model can improve its prognostic performance. Traditional regression models such as logistic regression, however, are limited due to overfitting when several covariates are included [10]; this implies that regression models fit the training cohort well, but they cannot be generalized to sufficiently reflect real-world cases. Moreover, variable selection is important if a high-dimensional feature exists [11].
In the least absolute shrinkage and selection operator (LASSO) regression model, the estimates of the regression coefficients are sparse, which implies that many components have exactly zero values. Thus, LASSO automatically deletes unnecessary covariates. LASSO has many desirable properties for regression models with several covariates.
The present study aimed to develop and validate a comprehensive predictive model to better stratify kidney transplant recipients according to DGF risk. We used the LASSO-logistic regression method to select suitable covariates from a vast amount of clinical and histological data obtained from pre-transplantation biopsy of kidney allografts. We also investigated the gain in the accuracy of the comprehensive nomogram model by incorporating histological signature and clinical risk factors for the preoperative prediction of DGF.
Materials and methods
Patients and ethical approval
This retrospective study was approved by the Ethics Committee of the First Affiliated Hospital of ** DGF before transplantation surgery is crucial as DGF is associated with adverse outcomes [19]. First, the occurrence of DGF increases the risk of acute rejection and long-term graft loss [20]. Second, DGF prolongs hospitalization, resulting in additional financial burden [20]. In a 5-year study of patients following kidney transplantation, early re-hospitalization after transplantation was a common event, occurring in 32% of the cohort, and only a few (9%) of these events had evidence of prevention. The leading causes of readmissions included surgical complications (15%), rejection (14%), volume metastasis (11%), and systemic and surgical wound infections (11% and 2.5%, respectively). Only 19 cases of rehospitalization (8%) met the criteria for prevention. The causes of early rehospitalization were varied, and the quality index after renal transplantation was also low [21].
Presently, the pathogenetic mechanisms of DGF remain unknown, and it is generally believed that both immune and nonimmune factors promote the occurrence and development of DGF [22]. These factors include donor-related factors (ischemia duration, age, kidney donor profile index [KDPI], end-stage sCr, history of hypertension, and CPR history), receptor-related factors (immune response, ischemia-reperfusion injury, and dialysis duration), and surgery-related factors [23]. The combined effect of these factors increases the allogenic response of organs, thus affecting their long-term survival. On the basis of etiology, predisposition, and underlying mechanisms, it is reasonable to infer that there are different subtypes of DGF, and consequently, the prognosis varies [24]. A recent study [25] reported commonly accepted risk factors for DGF, which included donation after cardiac death (DCD) donors, long CIT, long-distance transportation, pre-transplant dialysis recipients, past transplant recipients, diabetic recipients and higher BMI, longer waiting time, and donor-recipient mismatch. DGF is associated with an increased incidence of acute rejection and with poor long-term transplant outcomes. It is also associated with a lower quality of life. DGF leads to an increased financial burden; in a study based on the Premier Healthcare database, DGF was found to be associated with an average cost increase of approximately $18,000, an additional 6-day stay in the hospital, and an additional 2-day stay in the intensive care unit. Finally, together with the financial burden on the healthcare system, patients with DGF are also socially and psychologically affected, as they are often overwhelmed by the frequency of outpatient visits and their absence from home.
Currently, the accurate prediction of DGF is difficult to achieve; however, it is possible to identify high-risk DGF recipients at an early stage on the basis of a range of clinical and laboratory indicators. Our study included pathological and clinical data of 492 deceased donor (DD) kidney transplantation cases. The variables were selected by LASSO regression, and the multivariate logistic regression model with DGF as the endpoint was finally established. The nomogram of the model was drawn, and the practicability of the prediction model for evaluating donor kidney quality was assessed and verified.
Donor and recipient characteristics have significant implications for DGF prediction. In the last decade, Irish 2010 [9], Nyberg 2001 [26], Jeldres 2009 [19], and Chapal 2014 [27] conducted studies on the DGF predictive model. The variables included in each scoring system were different. The main DGF risk factors reported in these studies included donor age, BMI, terminal SCr level, cause of death, hypertension history, diabetes history, WIT, CIT, recipient BMI, HLA mismatch, PRA level, dialysis method and duration, and induction therapy method. In the present study, we extracted these risk factors and analyzed their correlation with DGF through the univariate analysis.
In our present study, CIT, recipient BMI, HLA mismatch, and dialysis method and duration were not correlated with DGF occurrence, mainly because of the following reasons. The recipients in China had a shorter waiting time before transplantation surgery. According to the 2015 Annual Chronic Kidney Disease Report in China [28], the median waiting time of domestic patients with uremia was 17.53 months, and approximately 40% of the recipients received kidney transplant surgery within 1 year after entering the waiting list. However, the median waiting time in USA for kidney transplant was 49.2 months [29]. Relatively, patients with uremia in China have shorter waiting time for kidney transplant and shorter dialysis time before transplantation. Uremia is a wasting disease. Longer waiting time and dialysis duration will deteriorate patients’ condition and decrease their tolerance to surgery, thereby resulting in a high risk of postoperative complications such as DGF and PNF. The variations in national and demographic characteristics can explain the differential risk factors of DGF to some extent.
When constructing the predictive model, it is worth noting that the effect of CIT on DGF is not significant, contrary to some previous studies [30]. In recent years, with the advancement of medicine and the deepening of DGF research, some researchers have found that the influence of CIT on DGF is gradually diminishing. For example, the survival rates of animal models of allografts treated with hydrogen sulfide in cold storage were significantly improved compared to controls (P < 0.01) [31]. In a large clinical database, also found that recipients using immunosuppressants after KT could also disregard the effects of CIT [27]. In analyzing data on 90,810 recipients of DD in the United States from 2010 to September 2018, reported that the risk of CIT for DGF was not significant [27]. Advances in the preservation of donor kidneys during transport, such as LifePort cryogenic machine perfusion, have significantly reduced the impact of CIT on renal ischemia-reperfusion injury [32]. Therefore, the series of trials and clinical cohort analyses described above suggest that central CIT has little significance for predicting DGF effects. In addition, using the original database, we found that the gender ratios of donors and recipients were highly imbalanced. This is related to the insufficient coverage of social ideology, family income and hospital propaganda. This imbalance will lead to significant errors in the model.
Previous studies have shown that HMP is beneficial and leads to significantly lower risk of DGF [33]. However, in the present study, no significant difference in DGF risk was observed between HMP-preserved donor kidneys and SCS-preserved donor kidneys; this might be due to the small number of SCS-preserved donor kidneys. The present study analyzed the correlation between HMP parameters and DGF. The results showed that the initial and terminal perfusion pressure, flow rate, and resistance parameters were significantly correlated with DGF; however, the difference in various parameters before and after perfusion was not significantly correlated with DGF. This study included a larger number of patients than a previous study and added the influence of Banff score and HMP on results. This finding was consistent with the results of previous research [8, 34,35,36].
Most chronic and acute histologic lesions in pre-transplant biopsy are independent risk factors of DGF; however, DGF predictive models have rarely included these histologic factors. The most important and final argument for using the nomogram is based on the combination of pre-transplant biopsies with donor clinical characteristics and HMP parameters. By performing variable selection through LASSO regression, this nomogram was significantly better than previous models reported in the above-mentioned literature [8, 34, 35]. The AUC values of this nomogram were 0.83 and 0.87 in the development and validation cohorts, respectively. These results demonstrate the potential utility of this model to predict patients at risk of develo** DGF. The old model was compared with the current nomogram model to reflect the current era of kidney transplantation. The study population was refined to improve the prediction accuracy of the model, which can also be used to predict long-term graft survival before transplantation.
The established DGF risk prediction model derived and validated a potential clinical prediction tool rather than a decision rule. In this study, chronic and acute lesions in pretransplant biopsies were assessed by semi-quantitative methods. There were significant differences in the distribution of chronic and acute lesions between the DGF and non-DGF groups, except for arterial hyaline degeneration. The pre-transplantation biopsy score can be used to inform clinicians’ evidence-based decision making regarding the use of kidneys to guide the management of kidney transplantation. For example, based on the mildly impaired and moderately injured, we recommend that the DCD kidney can be used with minimal risk of DGF. However, at severe damage, we recommend being cautious in the application of the DCD kidney and should be used in specific clinical situations. The DGF risk prediction model established in this study has reference value for the selection of kidney transplant donors and can be used to predict DGF before organ donation acquisition.
The limitations of the present study include small sample size and single center study. For better disease prediction, the sample size should be increased to increase the accuracy of prediction. Additionally, future studies should include multiple centers to further validate the clinical application of this nomogram.
Conclusion
In conclusion, this study presents a DGF prediction nomogram that incorporates donor clinical characteristics, HMP parameters, and pre-transplant biopsy features and can be conveniently used for preoperative individualized prediction of DGF in recipients before kidney transplantation surgery.
Data availability
The datasets generated and analysed during the current study are not publicly available due the datasets are private to the patients and the institution but are available from the corresponding author on reasonable request.
Code Availability
R code can be available by contacting authors.
Change history
18 June 2024
A Correction to this paper has been published: https://doi.org/10.1186/s12882-024-03641-8
References
Schröppel B, Legendre C. Delayed kidney graft function: from mechanism to translation. Kidney Int. 2014;86(2):251–8.
Wang CJ, et al. Association of Slow Graft Function with long-term outcomes in kidney transplant recipients. Ann Transpl. 2018;23:224–31.
Matas AJ, et al. OPTN/SRTR 2012 Annual Data Report: kidney. Am J Transpl. 2014;14(Suppl 1):11–44.
Hart A, et al. OPTN/SRTR 2018 Annual Data Report: kidney. Am J Transpl. 2020;20(Suppl s1):20–130.
Kramer A et al. The European Renal Association - European Dialysis and Transplant Association (ERA-EDTA) Registry Annual Report 2016: a summary Clin Kidney J, 2019. 12(5): p. 702–720.
Hu XJ, et al. Prediction of kidney transplant outcome based on different DGF definitions in Chinese deceased donation. BMC Nephrol. 2019;20(1):409.
Wang CJ, et al. The donor kidney biopsy and its implications in Predicting Graft outcomes: a systematic review. Am J Transpl. 2015;15(7):1903–14.
Ding CG, et al. Predictive score model for delayed graft function based on hypothermic machine perfusion variables in kidney transplantation. Chin Med J (Engl). 2018;131(22):2651–7.
Irish WD, et al. A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. Am J Transpl. 2010;10(10):2279–86.
Zhang Y, et al. Logistic bayesian LASSO for genetic association analysis of data from complex sampling designs. J Hum Genet. 2017;62(9):819–29.
Laurin C, Boomsma D, Lubke G. The use of vector bootstrap** to improve variable selection precision in Lasso models. Stat Appl Genet Mol Biol. 2016;15(4):305–20.
Zheng J, et al. Comprehensive assessment of deceased donor kidneys with clinical characteristics, pre-implant biopsy histopathology and hypothermic mechanical perfusion parameters is highly predictive of delayed graft function. Ren Fail. 2020;42(1):369–76.
Grosso G, et al. Delayed graft function and long-term outcome in kidney transplantation. Transpl Proc. 2012;44(7):1879–83.
Roufosse C et al. The Banff 2022 kidney Meeting Work Plan: Data-driven refinement of the Banff classification for renal allografts. Am J Transpl, 2023.
Remuzzi G, et al. Early experience with dual kidney transplantation in adults using expanded donor criteria. Double Kidney Transplant Group (DKG). J Am Soc Nephrol. 1999;10(12):2591–8.
Schneeweiss S, et al. Variable selection for Confounding Adjustment in High-dimensional Covariate spaces when analyzing Healthcare databases. Epidemiology. 2017;28(2):237–48.
Vickers AJ, et al. Extensions to decision curve analysis, a novel method for evaluating diagnostic tests, prediction models and molecular markers. BMC Med Inf Decis Mak. 2008;8:53.
Liang W, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1081–9.
Jeldres C, et al. Prediction of delayed graft function after renal transplantation. Can Urol Assoc J. 2009;3(5):377–82.
Mikhalski D, et al. Cold ischemia is a major determinant of acute rejection and renal graft survival in the modern era of immunosuppression. Transplantation. 2008;85(7 Suppl):S3–9.
Harhay M, et al. Early rehospitalization after kidney transplantation: assessing preventability and prognosis. Am J Transpl. 2013;13(12):3164–72.
Bahl D, et al. Delayed graft function in kidney transplantation. Curr Opin Organ Transpl. 2019;24(1):82–6.
Mezzolla V, et al. Emerging biomarkers of delayed graft function in kidney transplantation. Transpl Rev (Orlando). 2021;35(4):100629.
Zhang H, et al. Risk factors and outcomes of prolonged recovery from delayed graft function after deceased kidney transplantation. Ren Fail. 2020;42(1):792–8.
Yousif EAI, et al. In kidney recipients from the same deceased donor, discordance in delayed graft function is associated with the worst outcomes. Clin Transpl. 2022;36(9):e14779.
Nyberg SL, et al. Donor scoring system for cadaveric renal transplantation. Am J Transpl. 2001;1(2):162–70.
Chapal M, et al. A useful scoring system for the prediction and management of delayed graft function following kidney transplantation from cadaveric donors. Kidney Int. 2014;86(6):1130–9.
Zhang L et al. China Kidney Disease Network (CK-NET) 2015 Annual Data Report Kidney Int Suppl (2011), 2019. 9(1): p. e1-e81.
Smith JM, et al. OPTN/SRTR 2011 Annual Data Report: intestine. Am J Transpl. 2013;13(Suppl 1):103–18.
Melih KV, et al. Incidence, risk factors, and outcomes of delayed graft function in deceased donor kidney transplantation. Transpl Proc. 2019;51(4):1096–100.
Lobb I, et al. Hydrogen Sulfide protects renal grafts against prolonged Cold Ischemia-Reperfusion Injury via specific mitochondrial actions. Am J Transpl. 2017;17(2):341–52.
Hosgood SA, et al. A comparison of hypothermic machine perfusion versus static cold storage in an experimental model of renal ischemia reperfusion injury. Transplantation. 2010;89(7):830–7.
Domínguez J, et al. Factors that predict duration of delayed graft function in cadaveric kidney transplantation. Transpl Proc. 2009;41(6):2668–9.
Bissolati M, et al. Hypothermic machine perfusion as an alternative to Biopsy Assessment in transplantation of kidneys donated after Cardiocirculatory Death: a pilot study. Transpl Proc. 2019;51(9):2890–8.
De Deken J, Kocabayoglu P, Moers C. Hypothermic machine perfusion in kidney transplantation. Curr Opin Organ Transpl. 2016;21(3):294–300.
Pan J, Liao G. Development and validation of Nomogram for Predicting delayed graft function after kidney transplantation of deceased Donor. Int J Gen Med. 2021;14:9103–15.
Acknowledgements
Thanks are due to Yang Jian and Yan Bin of Clinical Research Center of First Affiliated Hospital of **’an Jiaotong University for assistance with the data analysis and the valuable discussion.
Funding
This study was supported by the This research was supported financially by the National Natural Science Foundation of China (NSFC; No. 82170768), the Natural Science Basic Research Program of Shaanxi Province (No. 2022JM-459), the Key R&D Plan of Shaanxi Province (No. 2021LL-JB-06) and the protective effect of mechanical perfusion combined with DRSAB on renal IRI and the molecular mechanism of reducing DGF and AR in transplanted kidney,Research project funding agreement of China Organ Transplantation Development Foundation (Protective effect of machine perfusion combined with DRSAb on renal IRI and its molecular mechanism).
Author information
Authors and Affiliations
Contributions
Conception and design: Y.L., J.Z., G.C., C.D. Collection and assembly of data: X.T., P.T., H.X., X.P., X.D. Data analysis and interpretation: M.L., X.H., Y.L., J.Z., W.X. Manuscript writing: M.L., X.H.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures were in accordance with the Helsinki Declaration. The protocol and consent form were approved by the Ethics Committee of First Affiliated Hospital of **’an Jiaotong University (No. XJTU1AF2015LSL-058). All patients were informed about the risk of pre-transplant biopsy and the objectives of the study and they all gave written informed consent. None of the participants denied the written consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: the authors identified that there are two duplicate names in the author's information, Chen-guang Ding and Chenguang Ding are the same person. We have deleted Chenguang Ding.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, M., Hu, X., Li, Y. et al. Development and validation of a novel nomogram model for predicting delayed graft function in deceased donor kidney transplantation based on pre-transplant biopsies. BMC Nephrol 25, 138 (2024). https://doi.org/10.1186/s12882-024-03557-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12882-024-03557-3